Tumour-derived transforming growth factor-β signalling contributes to fibrosis in patients with cancer cachexia
- PMID: 31273954
- PMCID: PMC6818454
- DOI: 10.1002/jcsm.12441
Tumour-derived transforming growth factor-β signalling contributes to fibrosis in patients with cancer cachexia
Abstract
Background: Cachexia is a paraneoplastic syndrome related with poor prognosis. The tumour micro-environment contributes to systemic inflammation and increased oxidative stress as well as to fibrosis. The aim of the present study was to characterise the inflammatory circulating factors and tumour micro-environment profile, as potentially contributing to tumour fibrosis in cachectic cancer patients.
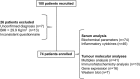
Methods: 74 patients (weight stable cancer n = 31; cachectic cancer n = 43) diagnosed with colorectal cancer were recruited, and tumour biopsies were collected during surgery. Multiplex assay was performed to study inflammatory cytokines and growth factors. Immunohistochemistry analysis was carried out to study extracellular matrix components.
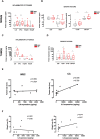
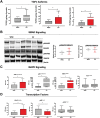
Results: Higher protein expression of inflammatory cytokines and growth factors such as epidermal growth factor, granulocyte-macrophage colony-stimulating factor, interferon-α, and interleukin (IL)-8 was observed in the tumour and serum of cachectic cancer patients in comparison with weight-stable counterparts. Also, IL-8 was positively correlated with weight loss in cachectic patients (P = 0.04; r = 0.627). Immunohistochemistry staining showed intense collagen deposition (P = 0.0006) and increased presence of α-smooth muscle actin (P < 0.0001) in tumours of cachectic cancer patients, characterizing fibrosis. In addition, higher transforming growth factor (TGF)-β1, TGF-β2, and TGF-β3 expression (P = 0.003, P = 0.05, and P = 0.047, respectively) was found in the tumour of cachectic patients, parallel to p38 mitogen-activated protein kinase alteration. Hypoxia-inducible factor-1α mRNA content was significantly increased in the tumour of cachectic patients, when compared with weight-stable group (P = 0.005).
Conclusions: Our results demonstrate TGF-β pathway activation in the tumour in cachexia, through the (non-canonical) mitogen-activated protein kinase pathway. The results show that during cachexia, intratumoural inflammatory response contributes to the onset of fibrosis. Tumour remodelling, probably by TGF-β-induced transdifferentiation of fibroblasts to myofibroblasts, induces unbalanced inflammatory cytokine profile, angiogenesis, and elevation of extracellular matrix components (EMC). We speculate that these changes may affect tumour aggressiveness and present consequences in peripheral organs.
Keywords: Cachexia; Epithelial-mesenchymal components; Fibrosis; Tumour micro-environment.
© 2019 The Authors Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
None declared.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- National Council for Scientific and Technological Development (CNPQ)/International
- Coordination for the Improvement of Higher Education Personnel (CAPES)/International
- 2016/08726-1/São Paulo Research Foundation/International
- 2012/50079-0/São Paulo Research Foundation/International
- 2016/04000-3/São Paulo Research Foundation/International
LinkOut - more resources
Full Text Sources

