Synergistic effects of a cremophor EL drug delivery system and its U0126 cargo in an ex vivo model
- PMID: 31274009
- PMCID: PMC6691891
- DOI: 10.1080/10717544.2019.1636421
Synergistic effects of a cremophor EL drug delivery system and its U0126 cargo in an ex vivo model
Abstract
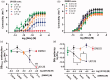
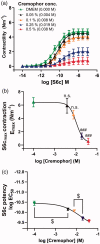
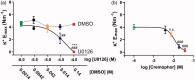
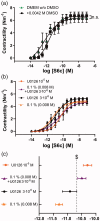
Neuroprotection has proven clinically unsuccessful in subarachnoid hemorrhage. We believe that this is because the major component in the early damage pathway, the vascular wall, has not been given the necessary focus. U0126 is a potent inhibitor of vascular phenotypical changes, exemplified by functional endothelin B (ETB) receptor upregulation. The current study aimed to determine the optimal dose of U0126 ex vivo and test the toxicology of this dose in vivo. To find the optimal dose and test a suitable in vivo delivery system, we applied an ex vivo model of blood flow cessation and investigated functional ETB receptor upregulation (using a specific agonist) as the primary endpoint. The secondary endpoint was depolarization-induced contractility assessed by 60 mM K+ stimuli. Furthermore, an in vivo toxicology study was performed on the optimal selected doses. U0126 (10 µM) had a strong effect on the prevention of functional ETB receptor contractility, combined with minimal effect on the depolarization-induced contractility. When cremophor EL was chosen for drug delivery, it had an inhibitory and additive effect (combined with U0126) on the ETB receptor contractility. Hence, 10 µM U0126 in 0.5% cremophor EL seems to be a dose that will be close to the maximal inhibition observed ex vivo on basilar arteries, without exhibiting side effects in the toxicology studies. U0126 and cremophor EL are well tolerated at doses that have effect on ETB receptor upregulation. Cremophor EL has an additional positive effect, preventing functional ETB receptor upregulation, making it suitable as a drug delivery system.
Keywords: Basilar artery; MEK1/2; U0126; cremophor EL; endothelin-1; optimal dose.
Figures

References
-
- Ahnstedt H, Stenman E, Cao L, et al. (2012). Cytokines and growth factors modify the upregulation of contractile endothelin ET(A) and ET(B) receptors in rat cerebral arteries after organ culture. Acta Physiol 205:266–78. - PubMed
-
- Ansar S, Larsen C, Maddahi A, Edvinsson L (2010). Subarachnoid hemorrhage induces enhanced expression of thromboxane A2 receptors in rat cerebral arteries. Brain Res 1316:163–72. - PubMed
-
- Ansar S, Vikman P, Nielsen M, et al. (2007). 5-HT1B, and AT1 receptor upregulation correlates with reduction in regional CBF after subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol 293:H3750–H3758. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
