Final analysis of a phase I/IIa trial of the folate-binding protein-derived E39 peptide vaccine to prevent recurrence in ovarian and endometrial cancer patients
- PMID: 31274231
- PMCID: PMC6712444
- DOI: 10.1002/cam4.2378
Final analysis of a phase I/IIa trial of the folate-binding protein-derived E39 peptide vaccine to prevent recurrence in ovarian and endometrial cancer patients
Abstract
Background: E39, an HLA-A2-restricted, immunogenic peptide derived from the folate-binding protein (FBP), is overexpressed in multiple malignancies. We conducted a phase I/IIa trial of the E39 + GM-CSF vaccine with booster inoculations of either E39 or E39' (an attenuated version of E39) to prevent recurrences in disease-free endometrial and ovarian cancer patients(pts). Here, we present the final 24-month landmark analysis.
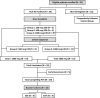
Patients and methods: HLA-A2 + patients receiving E39 + GM-CSF were included in the vaccine group (VG), and HLA-A2- pts (or HLA-A2 + patients refusing vaccine) were followed as the control group (CG). VG group received 6 monthly inoculations as the primary vaccine series (PVS) and were randomized to receive either E39 or E39' booster inoculations. Demographic, safety, immunologic, and disease-free survival (DFS) data were collected and evaluated.
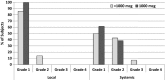
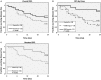
Results: Fifty-one patients were enrolled; 29 in the VG and 22 in the CG. Fourteen patients received <1000 μg and 15 received 1000 μg of E39. There were no clinicopathologic differences between VG and CG or between dose groups. E39 was well tolerated. At the 24 months landmark, DFS was 55.5% (VG) vs 40.0% (CG), P = 0.339. Patients receiving 1000 μg and boosted patients also showed improved DFS (P < 0.03). DFS was improved in the 1000 μg group after treatment of primary disease (90.0% vs CG:42.9%, P = 0.007), but not in recurrent patients. In low-FBP expressing patients, DFS was 100.0% (1000 μg), 50.0% (<1000 μg), and 25.0% (CG), P = 0.029.
Conclusions: This phase I/IIa trial reveals that E39 + GM-CSF is safe and may be effective in preventing recurrence in high-risk ovarian and endometrial cancer when optimally dosed (1000 μg) to FBP low patients being treated for primary disease.
Keywords: E39; FBP; endometrial cancer; immunotherapy; ovarian cancer; vaccine.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr George Peoples has partial inventor rights for the E39 and E39′ vaccines. The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of San Antonio Military Medical Center, the US Army Medical Department, US Air Force Medical Department, the Department of the Army, the Department of Air Force, Department of Defense or the US Government.
Figures


References
-
- Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression‐free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114(1):26‐31. - PubMed
-
- Peoples GE, Anderson BW, Fisk B, Kudelka AP, Wharton JT, Ioannides CG. Ovarian cancer‐associated lymphocyte recognition of folate binding protein peptides. Ann Surg Oncol. 1998;5(8):743‐750. - PubMed
-
- Li PY, DelVecchio S, Fonti R, et al. Local concentration of folate binding protein GP38 in sections of human ovarian carcinoma by in vitro quantitative autoradiography. J Nucl Med. 1996;37(4):665‐672. - PubMed
-
- Kim DK, Lee TV, Castilleja A, et al. Folate binding protein peptide 191–199 presented on dendritic cells can stimulate CTL from ovarian and breast cancer patients. Anticancer Res. 1999;19(4B):2907‐2916. - PubMed
-
- Hale DF, Vreeland TJ, Peoples GE. Arming the immune system through vaccination to prevent cancer recurrence. Am Soc Clin Oncol Educ Book. 2016;35:e159‐167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

