Secretion of Mast Cell Inflammatory Mediators Is Enhanced by CADM1-Dependent Adhesion to Sensory Neurons
- PMID: 31275114
- PMCID: PMC6591473
- DOI: 10.3389/fncel.2019.00262
Secretion of Mast Cell Inflammatory Mediators Is Enhanced by CADM1-Dependent Adhesion to Sensory Neurons
Abstract
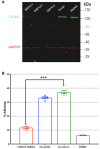
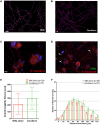
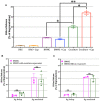
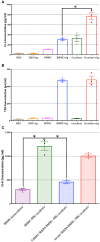
Neuroimmune interactions are important in the pathophysiology of many chronic inflammatory diseases, particularly those associated with alterations in sensory processing and pain. Mast cells and sensory neuron nerve endings are found in areas of the body exposed to the external environment, both are specialized to sense potential damage by injury or pathogens and signal to the immune system and nervous system, respectively, to elicit protective responses. Cell adhesion molecule 1 (CADM1), also known as SynCAM1, has previously been identified as an adhesion molecule which may couple mast cells to sensory neurons however, whether this molecule exerts a functional as well as structural role in neuroimmune cross-talk is unknown. Here we show, using a newly developed in vitro co-culture system consisting of murine bone marrow derived mast cells (BMMC) and adult sensory neurons isolated from dorsal root ganglions (DRG), that CADM1 is expressed in mast cells and adult sensory neurons and mediates strong adhesion between the two cell types. Non-neuronal cells in the DRG cultures did not express CADM1, and mast cells did not adhere to them. The interaction of BMMCs with sensory neurons was found to induce mast cell degranulation and IL-6 secretion and to enhance responses to antigen stimulation and activation of FcεRI receptors. Secretion of TNFα in contrast was not affected, nor was secretion evoked by compound 48/80. Co-cultures of BMMCs with HEK 293 cells, which also express CADM1, while also leading to adhesion did not replicate the effects of sensory neurons on mast cells, indicative of a neuron-specific interaction. Application of a CADM1 blocking peptide or knockdown of CADM1 in BMMCs significantly decreased BMMC attachment to sensory neurites and abolished the enhanced secretory responses of mast cells. In conclusion, CADM1 is necessary and sufficient to drive mast cell-sensory neuron adhesion and promote the development of a microenvironment in which neurons enhance mast cell responsiveness to antigen, this interaction could explain why the incidence of painful neuroinflammatory disorders such as irritable bowel syndrome (IBS) are increased in atopic patients.
Keywords: CADM1; IGE receptor; allergy; mast cells; pain; sensory neurons; synCAM1.
Figures





Similar articles
-
Increased expression of cell adhesion molecule 1 by mast cells as a cause of enhanced nerve-mast cell interaction in a hapten-induced mouse model of atopic dermatitis.Br J Dermatol. 2013 Apr;168(4):771-8. doi: 10.1111/bjd.12108. Br J Dermatol. 2013. PMID: 23106683
-
Cell adhesion molecule 1 (CADM1) on mast cells promotes interaction with dorsal root ganglion neurites by heterophilic binding to nectin-3.J Neuroimmunol. 2012 Sep 15;250(1-2):50-8. doi: 10.1016/j.jneuroim.2012.05.016. Epub 2012 Jun 15. J Neuroimmunol. 2012. PMID: 22703826
-
Enhanced nerve-mast cell interaction by a neuronal short isoform of cell adhesion molecule-1.J Immunol. 2011 May 15;186(10):5983-92. doi: 10.4049/jimmunol.1002244. Epub 2011 Apr 11. J Immunol. 2011. PMID: 21482734
-
Roles of Neuronal TRP Channels in Neuroimmune Interactions.In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 15. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 15. PMID: 29356476 Free Books & Documents. Review.
-
Nerve-mast cell and smooth muscle-mast cell interaction mediated by cell adhesion molecule-1, CADM1.J Smooth Muscle Res. 2008 Apr;44(2):83-93. doi: 10.1540/jsmr.44.83. J Smooth Muscle Res. 2008. PMID: 18552455 Review.
Cited by
-
Neuroimmune communication in allergic rhinitis.Front Neurol. 2023 Dec 21;14:1282130. doi: 10.3389/fneur.2023.1282130. eCollection 2023. Front Neurol. 2023. PMID: 38178883 Free PMC article. Review.
-
Polysialic Acid in the Immune System.Front Immunol. 2022 Feb 11;12:823637. doi: 10.3389/fimmu.2021.823637. eCollection 2021. Front Immunol. 2022. PMID: 35222358 Free PMC article. Review.
-
Resistance to local anesthesia in people with the Ehlers-Danlos Syndromes presenting for dental surgery.J Dent Anesth Pain Med. 2019 Oct;19(5):261-270. doi: 10.17245/jdapm.2019.19.5.261. Epub 2019 Oct 30. J Dent Anesth Pain Med. 2019. PMID: 31723666 Free PMC article.
-
FcεR1-expressing nociceptors trigger allergic airway inflammation.J Allergy Clin Immunol. 2021 Jun;147(6):2330-2342. doi: 10.1016/j.jaci.2020.12.644. Epub 2021 Jan 13. J Allergy Clin Immunol. 2021. PMID: 33453289 Free PMC article.
-
PTP-MEG2 regulates quantal size and fusion pore opening through two distinct structural bases and substrates.EMBO Rep. 2021 May 5;22(5):e52141. doi: 10.15252/embr.202052141. Epub 2021 Mar 25. EMBO Rep. 2021. PMID: 33764618 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

