On the Shape of the Force-Velocity Relationship in Skeletal Muscles: The Linear, the Hyperbolic, and the Double-Hyperbolic
- PMID: 31275173
- PMCID: PMC6593051
- DOI: 10.3389/fphys.2019.00769
On the Shape of the Force-Velocity Relationship in Skeletal Muscles: The Linear, the Hyperbolic, and the Double-Hyperbolic
Abstract
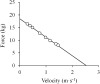
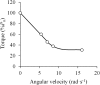
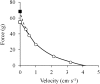
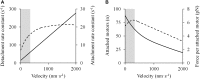
The shape of the force-velocity (F-V) relationship has important implications for different aspects of muscle physiology, such as muscle efficiency and fatigue, the understanding of the pathophysiology of several myopathies or the mechanisms of muscle contraction per se, and may be of relevance for other fields, such as the development of robotics and prosthetic applications featuring natural muscle-like properties. However, different opinions regarding the shape of the F-V relationship and the underlying mechanisms exist in the literature. In this review, we summarize relevant evidence on the shape of the F-V relationship obtained over the last century. Studies performed at multiple scales ranging from the sarcomere to the organism level have described the concentric F-V relationship as linear, hyperbolic or double-hyperbolic. While the F-V relationship has most frequently been described as a rectangular hyperbola, a large number of studies have found deviations from the hyperbolic function at both ends of the F-V relation. Indeed, current evidence suggests that the F-V relation in skeletal muscles follows a double-hyperbolic pattern, with a breakpoint located at very high forces/low velocities, which may be a direct consequence of the kinetic properties of myofilament cross-bridge formation. Deviations at low forces/high velocities, by contrast, may be related to a recently discovered, calcium-independent regulatory mechanism of muscle contraction, which may also explain the low metabolic cost of very fast muscle shortening contractions. Controversial results have also been reported regarding the eccentric F-V relationship, with studies in prepared muscle specimens suggesting that maximum eccentric force is substantially greater than isometric force, whereas in vivo studies in humans show only a modest increase, no change, or even a decrease in force in lengthening contractions. This review discusses possible reasons reported in the literature for these discrepant findings, including the testing procedures (familiarization, pre-load condition, and temperature) and a potential neural inhibition at higher lengthening velocities. Finally, some unresolved questions and recommendations for F-V testing in humans are reported at the end of this document.
Keywords: Edman’s equation; Hill’s equation; contraction velocity; maximal unloaded shortening velocity; motor unit; muscle contraction; muscle power; torque-velocity.
Figures



References
-
- Abbott B. C., Lowy J. (1956). J. Physiol. 133.∗ - PubMed
Publication types
LinkOut - more resources
Full Text Sources

