Overview of Current Therapeutics and Novel Candidates Against Influenza, Respiratory Syncytial Virus, and Middle East Respiratory Syndrome Coronavirus Infections
- PMID: 31275265
- PMCID: PMC6594388
- DOI: 10.3389/fmicb.2019.01327
Overview of Current Therapeutics and Novel Candidates Against Influenza, Respiratory Syncytial Virus, and Middle East Respiratory Syndrome Coronavirus Infections
Abstract
Emergence and re-emergence of respiratory virus infections represent a significant threat to global public health, as they occur seasonally and less frequently (such as in the case of influenza virus) as pandemic infections. Some of these viruses have been in the human population for centuries and others had recently emerged as a public health problem. Influenza viruses have been affecting the human population for a long time now; however, their ability to rapidly evolve through antigenic drift and antigenic shift causes the emergence of new strains. A recent example of these events is the avian-origin H7N9 influenza virus outbreak currently undergoing in China. Human H7N9 influenza viruses are resistant to amantadines and some strains are also resistant to neuraminidase inhibitors greatly limiting the options for treatment. Respiratory syncytial virus (RSV) may cause a lower respiratory tract infection characterized by bronchiolitis and pneumonia mainly in children and the elderly. Infection with RSV can cause severe disease and even death, imposing a severe burden for pediatric and geriatric health systems worldwide. Treatment for RSV is mainly supportive since the only approved therapy, a monoclonal antibody, is recommended for prophylactic use in high-risk patients. The Middle East respiratory syndrome coronavirus (MERS-CoV) is a newly emerging respiratory virus. The virus was first recognized in 2012 and it is associated with a lower respiratory tract disease that is more severe in patients with comorbidities. No licensed vaccines or antivirals have been yet approved for the treatment of MERS-CoV in humans. It is clear that the discovery and development of novel antivirals that can be used alone or in combination with existing therapies to treat these important respiratory viral infections are critical. In this review, we will describe some of the novel therapeutics currently under development for the treatment of these infections.
Keywords: MERS-coronavirus; antiviral; influenza; novel therapeutic agents; respiratory syncytial virus.
Figures
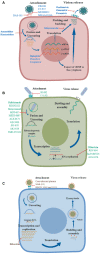
References
-
- Abarca K., Jung E., Fernández P., Zhao L., Harris B., Connor E. M., et al. . (2009). Safety, tolerability, pharmacokinetics, and immunogenicity of motavizumab, a humanized, enhanced-potency monoclonal antibody for the prevention of respiratory syncytial virus infection in at-risk children. Pediatr. Infect. Dis. J. 28, 267–272. 10.1097/INF.0b013e31818ffd03, PMID: - DOI - PubMed
-
- Ackermann M., Alnajjar S., Larios-Mora A., Koul A., Rigaux P., Gallup J., et al. (2016). “Therapeutic efficacy of JNJ-53718678, a RSV fusion inhibitor, in neonatal lambs” in 10th international respiratory syncytial virus symposium (Argentina: Patagonia; ).
-
- Al Ghamdi M., Alghamdi K. M., Ghandoora Y., Alzahrani A., Salah F., Alsulami A., et al. (2016). Treatment outcomes for patients with middle eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect. Dis. 16:174. 10.1186/s12879-016-1492-4 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

