Protease Activated Receptor 4 as a Novel Modulator of Regulatory T Cell Function
- PMID: 31275306
- PMCID: PMC6591367
- DOI: 10.3389/fimmu.2019.01311
Protease Activated Receptor 4 as a Novel Modulator of Regulatory T Cell Function
Abstract
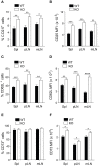
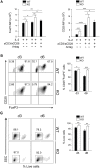
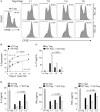
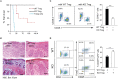
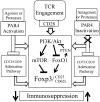
Regulatory T cells (Tregs) are a subpopulation of T cells that maintain immunological tolerance. In inflammatory responses the function of Tregs is tightly controlled by several factors including signaling through innate receptors such as Toll like receptors and anaphylatoxin receptors allowing an effective immune response to be generated. Protease-activated receptors (PARs) are another family of innate receptors expressed on multiple cell types and involved in the pathogenesis of autoimmune disorders. Whether proteases are able to directly modulate Treg function is unknown. Here, we show using two complimentary approaches that signaling through PAR-4 influences the expression of CD25, CD62L, and CD73, the suppressive capacity, and the stability of Tregs, via phosphorylation of FoxO1 and negative regulation of PTEN and FoxP3. Taken together, our results demonstrate an important role of PAR4 in tuning the function of Tregs and open the possibility of targeting PAR4 to modulate immune responses.
Keywords: PI3K/Akt signaling pathway; forkhead box protein O1 (FoxO1); immunoregulation; phosphatase and tensin homolog on chromosome 10 (PTEN); protease activated receptor 4 (PAR4); regulatory T cells (Tregs).
Figures


References
-
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. . Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. (1999) 162:5317–26. - PubMed
-
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, et al. . Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. (1998) 10:1969–80. 10.1093/intimm/10.12.1969 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

