MicroRNA-155 Controls T Helper Cell Activation During Viral Infection
- PMID: 31275315
- PMCID: PMC6593301
- DOI: 10.3389/fimmu.2019.01367
MicroRNA-155 Controls T Helper Cell Activation During Viral Infection
Abstract
MicroRNA (miR) 155 has been implicated in the regulation of innate and adaptive immunity as well as autoimmune processes. Importantly, it has been shown to regulate several antiviral responses, but its contribution to the immune response against cytopathic viruses such as vesicular stomatitis virus (VSV) infections is not known. Using transgenic/recombinant VSV expressing ovalbumin, we show that miR-155 is crucially involved in regulating the T helper cell response against this virus. Our experiments indicate that miR-155 in CD4+ T cells controls their activation, proliferation, and cytokine production in vitro and in vivo upon immunization with OVA as well as during VSV viral infection. Using intravital multiphoton microscopy we analyzed the interaction of antigen presenting cells (APCs) and T cells after OVA immunization and found impaired complex formation when using miR-155 deficient CD4+ T cells compared to wildtype CD4+ T cells ex vivo. In contrast, miR-155 was dispensable for the maturation of myeloid APCs and for their T cell stimulatory capacity. Our data provide the first evidence that miR-155 is required for efficient CD4+ T cell activation during anti-viral defense by allowing robust APC-T cell interaction required for activation and cytokine production of virus specific T cells.
Keywords: APC-T cell interaction; APCs; T cell activation; T helper cells; antiviral immunity; microRNA-155.
Figures




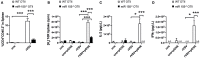
Similar articles
-
CD4(+) T cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability.J Exp Med. 2000 Jun 19;191(12):2159-70. doi: 10.1084/jem.191.12.2159. J Exp Med. 2000. PMID: 10859340 Free PMC article.
-
IL-12 is not required for induction of type 1 cytokine responses in viral infections.J Immunol. 1999 Jan 15;162(2):965-73. J Immunol. 1999. PMID: 9916721
-
Dendritic cell-independent B cell activation during acute virus infection: a role for early CCR7-driven B-T helper cell collaboration.J Immunol. 2007 Feb 1;178(3):1468-76. doi: 10.4049/jimmunol.178.3.1468. J Immunol. 2007. PMID: 17237395
-
Characterization of T-helper epitopes of the glycoprotein of vesicular stomatitis virus.J Virol. 1994 Mar;68(3):1573-80. doi: 10.1128/JVI.68.3.1573-1580.1994. J Virol. 1994. PMID: 7508998 Free PMC article.
-
T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction?Eur J Immunol. 1995 Dec;25(12):3445-51. doi: 10.1002/eji.1830251236. Eur J Immunol. 1995. PMID: 8566036
Cited by
-
Role of MicroRNAs in Host Defense against Infectious Bursal Disease Virus (IBDV) Infection: A Hidden Front Line.Viruses. 2020 May 14;12(5):543. doi: 10.3390/v12050543. Viruses. 2020. PMID: 32423052 Free PMC article. Review.
-
MiRNA Profiling in Plasma and Placenta of SARS-CoV-2-Infected Pregnant Women.Cells. 2021 Jul 15;10(7):1788. doi: 10.3390/cells10071788. Cells. 2021. PMID: 34359957 Free PMC article.
-
Airway mir-155 responses are associated with TH1 cytokine polarization in young children with viral respiratory infections.PLoS One. 2020 May 22;15(5):e0233352. doi: 10.1371/journal.pone.0233352. eCollection 2020. PLoS One. 2020. PMID: 32442188 Free PMC article.
-
The Role of miR-155 in Antitumor Immunity.Cancers (Basel). 2022 Nov 3;14(21):5414. doi: 10.3390/cancers14215414. Cancers (Basel). 2022. PMID: 36358832 Free PMC article. Review.
-
Role of miRNAs in Rheumatoid Arthritis Therapy.Cells. 2023 Jun 30;12(13):1749. doi: 10.3390/cells12131749. Cells. 2023. PMID: 37443783 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

