The Arabidopsis ATP-BINDING CASSETTE Transporter ABCB21 Regulates Auxin Levels in Cotyledons, the Root Pericycle, and Leaves
- PMID: 31275345
- PMCID: PMC6593225
- DOI: 10.3389/fpls.2019.00806
The Arabidopsis ATP-BINDING CASSETTE Transporter ABCB21 Regulates Auxin Levels in Cotyledons, the Root Pericycle, and Leaves
Erratum in
-
Corrigendum: The Arabidopsis ATP-BINDING CASSETTE Transporter ABCB21 Regulates Auxin Levels in Cotyledons, the Root Pericycle, and Leaves.Front Plant Sci. 2020 Apr 9;11:351. doi: 10.3389/fpls.2020.00351. eCollection 2020. Front Plant Sci. 2020. PMID: 32328075 Free PMC article.
Abstract
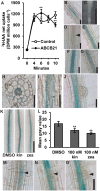
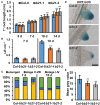
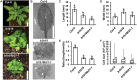
The phytohormone auxin plays significant roles in regulating plant growth and development. In Arabidopsis, a subset of ATP-BINDING CASSETTE subfamily B (ABCB) transporters participate in polar movement of auxin by exclusion from and prevention of reuptake at the plasma membrane. A previous analysis identified ABCB21 as a conditional auxin uptake/efflux transporter that regulates cellular auxin levels, but clear physiological roles for ABCB21 in planta remain unknown. Here we show that ABCB21 maintains the acropetal auxin transport stream by regulating auxin levels in the pericycle. Loss of ABCB21 reduces rootward auxin transport and delays lateral root emergence. In seedling shoots, ABCB21 regulates mobilization of auxin from the photosynthetic cotyledons that is important for phototropic bending. In rosette leaves ABCB21 contributes to lateral auxin distribution. These results support a primary role for ABCB21 in regulating auxin distribution supplementary to the primary ABCB auxin transporters ABCB1 and 19.
Keywords: ABCB transporter; Arabidopsis thaliana; auxin; development; seedling.
Figures






Similar articles
-
Arabidopsis ABCB21 is a facultative auxin importer/exporter regulated by cytoplasmic auxin concentration.Plant Cell Physiol. 2012 Dec;53(12):2090-100. doi: 10.1093/pcp/pcs149. Epub 2012 Nov 12. Plant Cell Physiol. 2012. PMID: 23147222
-
Corrigendum: The Arabidopsis ATP-BINDING CASSETTE Transporter ABCB21 Regulates Auxin Levels in Cotyledons, the Root Pericycle, and Leaves.Front Plant Sci. 2020 Apr 9;11:351. doi: 10.3389/fpls.2020.00351. eCollection 2020. Front Plant Sci. 2020. PMID: 32328075 Free PMC article.
-
Loss of Multiple ABCB Auxin Transporters Recapitulates the Major twisted dwarf 1 Phenotypes in Arabidopsis thaliana.Front Plant Sci. 2022 Apr 21;13:840260. doi: 10.3389/fpls.2022.840260. eCollection 2022. Front Plant Sci. 2022. PMID: 35528937 Free PMC article.
-
ABCB transporters: functionality extends to more than auxin transportation.Planta. 2025 Mar 18;261(4):93. doi: 10.1007/s00425-025-04662-9. Planta. 2025. PMID: 40100293 Review.
-
A Critical View on ABC Transporters and Their Interacting Partners in Auxin Transport.Plant Cell Physiol. 2017 Oct 1;58(10):1601-1614. doi: 10.1093/pcp/pcx104. Plant Cell Physiol. 2017. PMID: 29016918 Review.
Cited by
-
Improvement of a Genetic Transformation System and Preliminary Study on the Function of LpABCB21 and LpPILS7 Based on Somatic Embryogenesis in Lilium pumilum DC. Fisch.Int J Mol Sci. 2020 Sep 16;21(18):6784. doi: 10.3390/ijms21186784. Int J Mol Sci. 2020. PMID: 32947885 Free PMC article.
-
Understanding the Intricate Web of Phytohormone Signalling in Modulating Root System Architecture.Int J Mol Sci. 2021 May 24;22(11):5508. doi: 10.3390/ijms22115508. Int J Mol Sci. 2021. PMID: 34073675 Free PMC article. Review.
-
Differences and driving factors of leaf functional traits between old tree and mature tree of Pinus tabulaeformis in the Loess Plateau.BMC Plant Biol. 2025 Jan 30;25(1):129. doi: 10.1186/s12870-025-06130-8. BMC Plant Biol. 2025. PMID: 39885414 Free PMC article.
-
Characterization of the PIN Auxin Efflux Carrier Gene Family and Its Expression during Zygotic Embryogenesis in Persea americana.Plants (Basel). 2023 Jun 12;12(12):2280. doi: 10.3390/plants12122280. Plants (Basel). 2023. PMID: 37375905 Free PMC article.
-
Small compounds targeting tyrosine phosphorylation of Scaffold Protein Receptor for Activated C Kinase1A (RACK1A) regulate auxin mediated lateral root development in Arabidopsis.Plant Signal Behav. 2021 May 4;16(5):1899488. doi: 10.1080/15592324.2021.1899488. Epub 2021 Mar 30. Plant Signal Behav. 2021. PMID: 33784940 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

