Herbs-Partitioned Moxibustion Combined with Acupuncture Inhibits TGF- β 1-Smad-Snail-Induced Intestinal Epithelial Mesenchymal Transition in Crohn's Disease Model Rats
- PMID: 31275422
- PMCID: PMC6582898
- DOI: 10.1155/2019/8320250
Herbs-Partitioned Moxibustion Combined with Acupuncture Inhibits TGF- β 1-Smad-Snail-Induced Intestinal Epithelial Mesenchymal Transition in Crohn's Disease Model Rats
Abstract
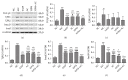
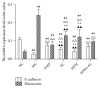
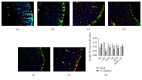
Crohn's disease may cause excessive damage and repair in the intestinal epithelium due to its chronic relapsing intestinal inflammation. These factors may initiate the TGF-β 1-Smad pathway to activate the transcription factor of Snail, and the Snail-mediated pathway promotes the transformation of intestinal epithelial cells to mesenchymal cells, leading to intestinal fibrosis. Acupuncture and moxibustion have been demonstrated to prevent intestinal fibrosis in Crohn's disease. However, it is not clear whether acupuncture and moxibustion can inhibit intestinal epithelial mesenchymal transformation in Crohn's disease by affecting the TGF-β 1-Smad-Snail pathway. This study indicated that abnormal increased expressions of TGFβ1, TβR2, Smad3, and Snail were significantly downregulated by herbs-partitioned moxibustion at Tianshu (ST25) and Qihai (RN6) and acupuncture at Zusanli (ST36) and Shangjuxu (ST37). In addition, protein and mRNA levels of E-cadherin, the epithelial cell marker, were significantly increased. Protein and mRNA levels of fibronectin, the mesenchymal cell marker, were decreased in the intestinal tissue. Moreover, the number of mesenchymal cells in the intestinal mucosa can be reversely transformed to intestinal epithelial cells. Therefore, herbs-partitioned moxibustion combined with acupuncture can prevent intestinal epithelial mesenchymal transition by inhibiting abnormal expression of TGFβ1, TβR2, Smad3, and Snail in the TGF-β1-Smad-Snail pathway in Crohn's disease.
Figures

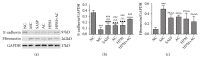
Similar articles
-
Acupuncture and Moxibustion Inhibited Intestinal Epithelial-Mesenchymal Transition in Patients with Crohn's Disease Induced by TGF- β 1/Smad3/Snail Pathway: A Clinical Trial Study.Chin J Integr Med. 2022 Sep;28(9):823-832. doi: 10.1007/s11655-022-2888-1. Epub 2022 Apr 13. Chin J Integr Med. 2022. PMID: 35419729 Clinical Trial.
-
Moxibustion combined with acupuncture increases tight junction protein expression in Crohn's disease patients.World J Gastroenterol. 2015 Apr 28;21(16):4986-96. doi: 10.3748/wjg.v21.i16.4986. World J Gastroenterol. 2015. PMID: 25945013 Free PMC article. Clinical Trial.
-
Qinggan Huoxue Recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-β1/Smad signaling pathway.World J Gastroenterol. 2016 May 21;22(19):4695-706. doi: 10.3748/wjg.v22.i19.4695. World J Gastroenterol. 2016. PMID: 27217701 Free PMC article.
-
[Progression and reflection on the mechanism study of acupuncture and moxibustion in treatment of Crohn's disease].Zhen Ci Yan Jiu. 2023 Feb 25;48(2):139-46. doi: 10.13702/j.1000-0607.20221037. Zhen Ci Yan Jiu. 2023. PMID: 36858409 Review. Chinese.
-
Acupuncture and moxibustion for treatment of Crohn's disease: A brief review.World J Gastroenterol. 2022 Jul 7;28(25):3001-3003. doi: 10.3748/wjg.v28.i25.3001. World J Gastroenterol. 2022. PMID: 35978879 Free PMC article. Review.
Cited by
-
Inflammation-fibrosis interplay in inflammatory bowel disease: mechanisms, progression, and therapeutic strategies.Front Pharmacol. 2025 Feb 28;16:1530797. doi: 10.3389/fphar.2025.1530797. eCollection 2025. Front Pharmacol. 2025. PMID: 40093318 Free PMC article. Review.
-
Effects of Electroacupuncture at Different Acupoints on Functional Dyspepsia Rats.Evid Based Complement Alternat Med. 2022 Feb 1;2022:6548623. doi: 10.1155/2022/6548623. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35154349 Free PMC article.
-
Herb-Partitioned Moxibustion Improves Crohn's Disease-Associated Intestinal Fibrosis by Suppressing the RhoA/ROCK1/MLC Pathway.Evid Based Complement Alternat Med. 2021 Nov 17;2021:2247953. doi: 10.1155/2021/2247953. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34840583 Free PMC article.
-
Acupuncture for Crohn's disease: a protocol for systematic review and meta-analysis.BMJ Open. 2023 Mar 15;13(3):e070578. doi: 10.1136/bmjopen-2022-070578. BMJ Open. 2023. PMID: 36921947 Free PMC article.
-
A Review on the Immunomodulatory Mechanism of Acupuncture in the Treatment of Inflammatory Bowel Disease.Evid Based Complement Alternat Med. 2022 Jan 15;2022:8528938. doi: 10.1155/2022/8528938. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35075366 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

