EGFR-AS1/HIF2A regulates the expression of FOXP3 to impact the cancer stemness of smoking-related non-small cell lung cancer
- PMID: 31275431
- PMCID: PMC6598324
- DOI: 10.1177/1758835919855228
EGFR-AS1/HIF2A regulates the expression of FOXP3 to impact the cancer stemness of smoking-related non-small cell lung cancer
Abstract
Background: Early data showed that FOXP3 could induce epithelial-mesenchymal transition by stimulating the Wnt/β-catenin signaling pathway in non-small cell lung cancer (NSCLC). However, how the expression of FOXP3 is regulated in NSCLC remains unknown. We thus explored the impacts of the long noncoding RNA EGFR antisense RNA 1 (EGFR-AS1) and hypoxia-inducible factor-2A (HIF2A) on FOXP3 expression and the cancer stemness of NSCLC.
Methods: Lung tissues samples from 87 patients with NSCLC and two NSCLC cell lines were used in this study. The regulation of FOXP3 and lung cancer cell stemness by EGFR-AS1 and HIF2A was determined at molecular levels in NSCLC tissue samples and cultured cells in the presence/absence of the smoking carcinogen, 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (also known as nicotine-derived nitrosamine ketone). The results were confirmed in tumor xenograft models.
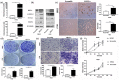
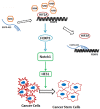
Results: We found that NNK decreased the expression of EGFR-AS1 in the long term, but increased the expression of HIF2A and FOXP3 to stimulate lung cancer cell stemness. EGFR-AS1 significantly inhibited FOXP3 expression and NSCLC cell stemness, whereas HIF2A obviously promoted both. The enhancement of lung cancer stemness by FOXP3 was, at least partially, via stimulating Notch1, as the inhibition of Notch1 could markedly diminish the effect of FOXP3.
Conclusions: FOXP3, the expression of which is under the fine control of EGFR-AS1, is a critical molecule that promotes NSCLC cancer cell stemness through stimulating the Notch1 pathway.
Keywords: EGFR antisense RNA 1; FOXP3; cancer cell stemness; hypoxia-inducible factor-2 alpha; non-small cell lung cancer.
Conflict of interest statement
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Figures





Similar articles
-
LncRNA FEZF1-AS1 enhances epithelial-mesenchymal transition (EMT) through suppressing E-cadherin and regulating WNT pathway in non-small cell lung cancer (NSCLC).Biomed Pharmacother. 2017 Nov;95:331-338. doi: 10.1016/j.biopha.2017.08.057. Epub 2017 Sep 12. Biomed Pharmacother. 2017. PMID: 28858731
-
Long noncoding RNA MACC1-AS1 promotes the stemness of nonsmall cell lung cancer cells through promoting UPF1-mediated destabilization of LATS1/2.Environ Toxicol. 2020 Sep;35(9):998-1006. doi: 10.1002/tox.22936. Epub 2020 May 13. Environ Toxicol. 2020. PMID: 32401390
-
Glioma-associated oncogene homolog 1 stimulates FOXP3 to promote non-small cell lung cancer stemness.Am J Transl Res. 2020 May 15;12(5):1839-1850. eCollection 2020. Am J Transl Res. 2020. PMID: 32509180 Free PMC article.
-
LncRNA DHRS4-AS1 Inhibits the Stemness of NSCLC Cells by Sponging miR-224-3p and Upregulating TP53 and TET1.Front Cell Dev Biol. 2020 Dec 23;8:585251. doi: 10.3389/fcell.2020.585251. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33425890 Free PMC article.
-
FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer.Mol Cancer. 2017 Jul 17;16(1):124. doi: 10.1186/s12943-017-0700-1. Mol Cancer. 2017. PMID: 28716029 Free PMC article.
Cited by
-
Regulation of Notch1 Signalling by Long Non-Coding RNAs in Cancers and Other Health Disorders.Int J Mol Sci. 2023 Aug 8;24(16):12579. doi: 10.3390/ijms241612579. Int J Mol Sci. 2023. PMID: 37628760 Free PMC article. Review.
-
Study on the mechanism by which Xuanfu Hua Tang increases sensitivity of hepatocellular carcinoma cells to sorafenib by antagonizing the Notch1 pathway through HIF-2α.Front Oncol. 2025 May 15;15:1552480. doi: 10.3389/fonc.2025.1552480. eCollection 2025. Front Oncol. 2025. PMID: 40444091 Free PMC article.
-
FOXP3: A Player of Immunogenetic Architecture in Lung Cancer.Genes (Basel). 2024 Apr 15;15(4):493. doi: 10.3390/genes15040493. Genes (Basel). 2024. PMID: 38674427 Free PMC article. Review.
-
Clinical role of the long non‑coding RNA, EGFR‑AS1, in patients with cancer: A systematic review and meta‑analysis.Oncol Lett. 2024 Mar 8;27(5):199. doi: 10.3892/ol.2024.14332. eCollection 2024 May. Oncol Lett. 2024. PMID: 38516689 Free PMC article.
-
Wnt signaling and tumors (Review).Mol Clin Oncol. 2024 May 15;21(1):45. doi: 10.3892/mco.2024.2743. eCollection 2024 Jul. Mol Clin Oncol. 2024. PMID: 38798312 Free PMC article. Review.
References
-
- Heng WS, Gosens R, Kruyt FAE. Lung cancer stem cells: origin, features, maintenance mechanisms and therapeutic targeting. Biochem Pharmacol 2019; 160: 121–133. - PubMed
-
- Liu Y, Yang SC, Li MY, et al. Tumorigenesis of smoking carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is related to its ability to stimulate thromboxane synthase and enhance stemness of non-small cell lung cancer stem cells. Cancer Lett 2016; 370: 198–206. - PubMed
-
- Huang WC, Kuo KT, Adebayo BO, et al. Garcinol inhibits cancer stem cell-like phenotype via suppression of the Wnt/Beta-catenin/STAT3 axis signalling pathway in human non-small cell lung carcinomas. J Nutr Biochem 2018; 54: 140–150. - PubMed
-
- Liu T, Wu X, Chen T, et al. Downregulation of DNMT3a by MIR-708–5p inhibits lung cancer stem cell-like phenotypes through repressing Wnt/Beta-catenin signaling. Clin Cancer Res 2018; 24: 1748–1760. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

