Metabolomic and biochemical characterization of a new model of the transition of acute kidney injury to chronic kidney disease induced by folic acid
- PMID: 31275747
- PMCID: PMC6590474
- DOI: 10.7717/peerj.7113
Metabolomic and biochemical characterization of a new model of the transition of acute kidney injury to chronic kidney disease induced by folic acid
Abstract
Background: Renal diseases represent a major public health problem. The demonstration that maladaptive repair of acute kidney injury (AKI) can lead to the development of chronic kidney disease (CKD) and end-stage renal disease has generated interest in studying the pathophysiological pathways involved. Animal models of AKI-CKD transition represent important tools to study this pathology. We hypothesized that the administration of multiple doses of folic acid (FA) would lead to a progressive loss of renal function that could be characterized through biochemical parameters, histological classification and nuclear magnetic resonance (NMR) profiling.
Methods: Wistar rats were divided into groups: the control group received a daily intraperitoneal (I.P.) injection of double-distilled water, the experimental group received a daily I.P. injection of FA (250 mg kg body weight-1). Disease was classified according to blood urea nitrogen level: mild (40-80 mg dL-1), moderate (100-200 mg dL-1) and severe (>200 mg dL-1). We analyzed through biochemical parameters, histological classification and NMR profiling.
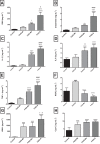
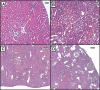
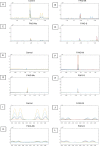
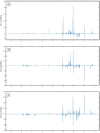
Results: Biochemical markers, pro-inflammatory cytokines and kidney injury biomarkers differed significantly (P < 0.05) between control and experimental groups. Histology revealed that as damage progressed, the degree of tubular injury increased, and the inflammatory infiltrate was more evident. NMR metabolomics and chemometrics revealed differences in urinary metabolites associated with CKD progression. The main physiological pathways affected were those involved in energy production and amino-acid metabolism, together with organic osmolytes. These data suggest that multiple administrations of FA induce a reproducible model of the induction of CKD. This model could help to evaluate new strategies for nephroprotection that could be applied in the clinic.
Keywords: Acute kidney disease; Chronic kidney disease; Folic acid; NMR metabolomics; PCA.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Agharazii M, St-Louis R, Gautier-Bastien A, Ung R-V, Mokas S, Lariviere R, Richard DE. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. American Journal of Hypertension. 2015;28(6):746–755. doi: 10.1093/ajh/hpu225. - DOI - PubMed
-
- Al Za’abi M, Al Salam S, Al Suleimani Y, Manoj P, Nemmar A, Ali BH. Gum acacia improves renal function and ameliorates systemic inflammation, oxidative and nitrosative stress in streptozotocin-induced diabetes in rats with adenine-induced chronic kidney disease. Cellular Physiology and Biochemistry. 2018;45(6):2293–2304. doi: 10.1159/000488176. - DOI - PubMed
-
- Ali BH, Al-Salam S, Al Suleimani Y, Al Kalbani J, Al Bahlani S, Ashique M, Manoj P, Al Dhahli B, Al Abri N, Naser HT, Yasin J, Nemmar A, Al Za’abi M, Hartmann C, Schupp N. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic & Clinical Pharmacology & Toxicology. 2018;122(1):65–73. doi: 10.1111/bcpt.12817. - DOI - PubMed
LinkOut - more resources
Full Text Sources

