Identification of Antidiabetic Metabolites from Paederia foetida L. Twigs by Gas Chromatography-Mass Spectrometry-Based Metabolomics and Molecular Docking Study
- PMID: 31275982
- PMCID: PMC6560335
- DOI: 10.1155/2019/7603125
Identification of Antidiabetic Metabolites from Paederia foetida L. Twigs by Gas Chromatography-Mass Spectrometry-Based Metabolomics and Molecular Docking Study
Abstract
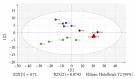
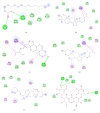
Paederia foetida L. (Rubiaceae) is a climber which is widely distributed in Asian countries including Malaysia. The plant is traditionally used to treat various diseases including diabetes. This study is to evaluate the enzymatic inhibition activity of Paederia foetida twigs extracts and to identify the metabolites responsible for the bioactivity by gas chromatography-mass spectrometry (GC-MS) metabolomics profiling. Three different twig extracts, namely, hexane (PFH), chloroform (PFC), and methanol (PFM), were submerged for their α-amylase and α-glucosidase inhibition potential in 5 replicates for each. Results obtained from the loading column scatter plot of orthogonal partial least square (OPLS) model revealed the presence of 12 bioactive compounds, namely, dl-α-tocopherol, n-hexadecanoic acid, 2-hexyl-1-decanol, stigmastanol, 2-nonadecanone, cholest-8(14)-en-3-ol, 4,4-dimethyl-, (3β,5α)-, stigmast-4-en-3-one, stigmasterol, 1-ethyl-1-tetradecyloxy-1-silacyclohexane, ɣ-sitosterol, stigmast-7-en-3-ol, (3β,5α,24S)-, and α-monostearin. In silico molecular docking was carried out using the crystal structure α-amylase (PDB ID: 4W93) and α-glucosidase (PDB ID: 3WY1). α-Amylase-n-hexadecanoic acid exhibited the lowest binding energy of -2.28 kcal/mol with two hydrogen bonds residue, namely, LYS178 and TYR174, along with hydrophobic interactions involving PRO140, TRP134, SER132, ASP135, and LYS172. The binding interactions of α-glucosidase-n-hexadecanoic acid complex ligand also showed the lowest binding energy among 5 major compounds with the energy value of -4.04 kcal/mol. The complex consists of one hydrogen bond interacting residue, ARG437, and hydrophobic interactions with ALA444, ASP141, GLN438, GLU432, GLY374, LEU373, LEU433, LYS352, PRO347, THR445, HIS348, and PRO351. The study provides informative data on the potential antidiabetic inhibitors identified in Paederia foetida twigs, indicating the plant has the therapeutic effect properties to manage diabetes.
Figures







References
-
- World Health Organization. Global Report on Diabetes. World Health Organization; 2016.
-
- Ahmed A. A., Islam M. M., Rahman M. A., Hossain M. A. Thrombolytic, cytotoxic and antidiabetic effects of Paederia foetida L. leaf extract. British Journal of Medicine & Medical Research. 2014;4(5):1244–1256.
-
- Upadhyaya S. Screening of phytochemicals, nutritional status, antioxidant and antimicrobial activity of Paederia foetida Linn. from different localities of Assam, India. Journal of Pharmacy Research. 2013;7(1):139–141. doi: 10.1016/j.jopr.2013.01.015. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

