Unravelling the role of amino acid sequence order in the assembly and function of the amyloid-β core
- PMID: 31276123
- PMCID: PMC7616937
- DOI: 10.1039/c9cc03654g
Unravelling the role of amino acid sequence order in the assembly and function of the amyloid-β core
Abstract
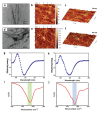
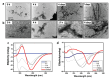
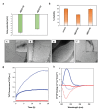
The amino acid sequence plays an essential role in amyloid formation. Here, using the central core recognition module of the Aβ peptide and its reverse sequence, we show that although both peptides assemble into β-sheets, their morphologies, kinetics and cell toxicities display marked differences. In addition, the native peptide, but not the reverse one, shows notable affinity towards bilayer lipid model membranes that modulates the aggregation pathways to stabilize the oligomeric intermediate states and function as the toxic agent responsible for neuronal dysfunction.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Membrane domain modulation of Aβ1-42 oligomer interactions with supported lipid bilayers: an atomic force microscopy investigation.Nanoscale. 2019 Nov 21;11(43):20857-20867. doi: 10.1039/c9nr06361g. Epub 2019 Oct 28. Nanoscale. 2019. PMID: 31657431
-
Different effects of Alzheimer's peptide Aβ(1-40) oligomers and fibrils on supported lipid membranes.Biophys Chem. 2013 Dec 1;182:23-9. doi: 10.1016/j.bpc.2013.07.010. Epub 2013 Aug 14. Biophys Chem. 2013. PMID: 23998637
-
Cholesterol modulates the interaction of beta-amyloid peptide with lipid bilayers.Biophys J. 2009 May 20;96(10):4299-307. doi: 10.1016/j.bpj.2009.02.036. Biophys J. 2009. PMID: 19450500 Free PMC article.
-
Vesicle-Based Assays to Study Membrane Interactions of Amyloid Peptides.Methods Mol Biol. 2019;1873:39-51. doi: 10.1007/978-1-4939-8820-4_3. Methods Mol Biol. 2019. PMID: 30341602 Review.
-
Conformations and biological activities of amyloid beta peptide 25-35.Curr Protein Pept Sci. 2010 Feb;11(1):54-67. doi: 10.2174/138920310790274626. Curr Protein Pept Sci. 2010. PMID: 20201807 Review.
Cited by
-
Cholesterol as a key player in amyloid β-mediated toxicity in Alzheimer's disease.Front Mol Neurosci. 2022 Aug 25;15:937056. doi: 10.3389/fnmol.2022.937056. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36090253 Free PMC article. Review.
-
Structure and Conservation of Amyloid Spines From the Candida albicans Als5 Adhesin.Front Mol Biosci. 2022 Jul 6;9:926959. doi: 10.3389/fmolb.2022.926959. eCollection 2022. Front Mol Biosci. 2022. PMID: 35874616 Free PMC article.
-
Helix Dipole and Membrane Electrostatics Delineate Conformational Transitions in the Self-Assembly of Amyloidogenic Peptides.Langmuir. 2020 Jul 28;36(29):8389-8397. doi: 10.1021/acs.langmuir.0c00723. Epub 2020 Jul 15. Langmuir. 2020. PMID: 32628488 Free PMC article.
-
Does the inclusion of electronic polarisability lead to a better modelling of peptide aggregation?RSC Adv. 2022 Jul 21;12(32):20829-20837. doi: 10.1039/d2ra01478e. eCollection 2022 Jul 14. RSC Adv. 2022. PMID: 35919139 Free PMC article.
-
Photocontrolled Reversible Amyloid Fibril Formation of Parathyroid Hormone-Derived Peptides.Bioconjug Chem. 2024 Jul 17;35(7):981-995. doi: 10.1021/acs.bioconjchem.4c00188. Epub 2024 Jun 12. Bioconjug Chem. 2024. PMID: 38865349 Free PMC article.
References
-
- Teplow DB. Amyloid. 1998;5:121. - PubMed
- Selkoe DJ. Nat Med. 2011;17:1060. - PubMed
- Chiti F, Dobson CM. Annu Rev Biochem. 2006;75:333. - PubMed
- Hamley IW. Chem Rev. 2012;112:5147. - PubMed
- Wei G, Su ZQ, Reynolds NP, Arosio P, Hamley IW, Gazit E, Mezzenga R. Chem Soc Rev. 2017;46:4661. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

