Rosiglitazone in the thawing medium improves mitochondrial function in stallion spermatozoa through regulating Akt phosphorylation and reduction of caspase 3
- PMID: 31276504
- PMCID: PMC6611560
- DOI: 10.1371/journal.pone.0211994
Rosiglitazone in the thawing medium improves mitochondrial function in stallion spermatozoa through regulating Akt phosphorylation and reduction of caspase 3
Abstract
Background: The population of stallion spermatozoa that survive thawing experience compromised mitochondrial functionality and accelerated senescence, among other changes. It is known that stallion spermatozoa show very active oxidative phosphorylation that may accelerate sperm senescence through increased production of reactive oxygen species. Rosiglitazone has been proven to enhance the glycolytic capability of stallion spermatozoa maintained at ambient temperature.
Objectives: Thus, we hypothesized that thawed sperm may also benefit from rosiglitazone supplementation.
Materials and methods: Thawed sperm were washed and resuspended in Tyrodes media, and the samples were divided and supplemented with 0 or 75 μM rosiglitazone. After one and two hours of incubation, mitochondrial functionality, Akt phosphorylation and caspase 3 activity were evaluated. Additional samples were incubated in the presence of an Akt1/2 inhibitor, compound C (an AMPK inhibitor) or GW9662 (an antagonist of the PPARγ receptor).
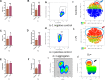
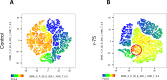
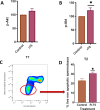
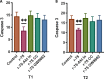
Results: Rosiglitazone maintained Akt phosphorylation and reduced caspase 3 activation (p<0.01), both of which were prevented by incubation in the presence of the three inhibitors. Rosiglitazone also enhanced mitochondrial functionality (P<0.01).
Conclusion: We provide the first evidence that the functionality of frozen stallion spermatozoa can be potentially improved after thawing through the activation of pro survival pathways, providing new clues for improving current sperm biotechnology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Rosiglitazone Improves Stallion Sperm Motility, ATP Content, and Mitochondrial Function.Biol Reprod. 2016 Nov;95(5):107. doi: 10.1095/biolreprod.116.142687. Epub 2016 Sep 28. Biol Reprod. 2016. PMID: 27683266
-
Phosphorylated AKT preserves stallion sperm viability and motility by inhibiting caspases 3 and 7.Reproduction. 2014 Aug;148(2):221-35. doi: 10.1530/REP-13-0191. Epub 2014 May 21. Reproduction. 2014. PMID: 24850868
-
Metformin and rosiglitazone affect motility, lipid peroxidation and mitochondrial activity of thawed equine spermatozoa.J Equine Vet Sci. 2025 Jun;149:105570. doi: 10.1016/j.jevs.2025.105570. Epub 2025 Apr 11. J Equine Vet Sci. 2025. PMID: 40222464
-
The Impact of Reproductive Technologies on Stallion Mitochondrial Function.Reprod Domest Anim. 2015 Aug;50(4):529-37. doi: 10.1111/rda.12551. Epub 2015 Jun 1. Reprod Domest Anim. 2015. PMID: 26031351 Review.
-
Commercial semen freezing: individual male variation in cryosurvival and the response of stallion sperm to customized freezing protocols.Anim Reprod Sci. 2008 Apr;105(1-2):119-28. doi: 10.1016/j.anireprosci.2007.11.010. Epub 2007 Nov 26. Anim Reprod Sci. 2008. PMID: 18178040 Review.
Cited by
-
Frequency of Semen Collection Affects Ram Sperm Cryoresistance.Animals (Basel). 2022 Jun 8;12(12):1492. doi: 10.3390/ani12121492. Animals (Basel). 2022. PMID: 35739829 Free PMC article.
-
Repairing Effect of Mesenchymal Stem Cells on Lead Acetate-Induced Testicular Injury in Mice.Cell Transplant. 2024 Jan-Dec;33:9636897231219395. doi: 10.1177/09636897231219395. Cell Transplant. 2024. PMID: 38173262 Free PMC article.
-
A Descriptive Study of Brown Bear (Ursus arctos) Sperm Quality and Proteomic Profiles Considering Sperm Origin.Animals (Basel). 2025 Jul 12;15(14):2064. doi: 10.3390/ani15142064. Animals (Basel). 2025. PMID: 40723528 Free PMC article.
-
The characterization of CellROX™ probes could be a crucial factor in ram sperm quality assessment.Front Vet Sci. 2024 Feb 27;11:1342808. doi: 10.3389/fvets.2024.1342808. eCollection 2024. Front Vet Sci. 2024. PMID: 38476170 Free PMC article.
-
Protective effect of rosiglitazone on microscopic and oxidative stress parameters of ram sperm after freeze-thawing.Sci Rep. 2022 Aug 17;12(1):13981. doi: 10.1038/s41598-022-18298-2. Sci Rep. 2022. PMID: 35978030 Free PMC article.
References
-
- Neagu VR, Garcia BM, Rodriguez AM, Ferrusola CO, Bolanos JM, Fernandez LG, et al. Determination of glutation peroxidase and superoxide dismutase activities in canine seminal plasma and its relation with sperm quality and lipid peroxidation post thaw. Theriogenology. 2011;75(1):10–6. 10.1016/j.theriogenology.2010.07.004 . - DOI - PubMed
-
- Graham JK. Cryopreservation of stallion spermatozoa. The Veterinary clinics of North America Equine practice. 1996;12(1):131–47. Epub 1996/04/01. . - PubMed
-
- Heitland AV, Jasko DJ, Squires EL, Graham JK, Pickett BW, Hamilton C. Factors affecting motion characteristics of frozen-thawed stallion spermatozoa. Equine veterinary journal. 1996;28(1):47–53. Epub 1996/01/01. . - PubMed
-
- Bedford SJ, Jasko DJ, Graham JK, Amann RP, Squires EL, Pickett BW. Effect of seminal extenders containing egg yolk and glycerol on motion characteristics and fertility of stallion spermatozoa. Theriogenology. 1995;43(5):955–67. Epub 1995/04/01. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

