Closing the DNA replication cycle: from simple circular molecules to supercoiled and knotted DNA catenanes
- PMID: 31276584
- PMCID: PMC6698734
- DOI: 10.1093/nar/gkz586
Closing the DNA replication cycle: from simple circular molecules to supercoiled and knotted DNA catenanes
Abstract
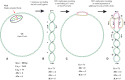
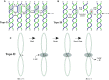
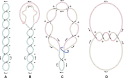
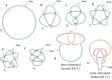
Due to helical structure of DNA, massive amounts of positive supercoils are constantly introduced ahead of each replication fork. Positive supercoiling inhibits progression of replication forks but various mechanisms evolved that permit very efficient relaxation of that positive supercoiling. Some of these mechanisms lead to interesting topological situations where DNA supercoiling, catenation and knotting coexist and influence each other in DNA molecules being replicated. Here, we first review fundamental aspects of DNA supercoiling, catenation and knotting when these qualitatively different topological states do not coexist in the same circular DNA but also when they are present at the same time in replicating DNA molecules. We also review differences between eukaryotic and prokaryotic cellular strategies that permit relaxation of positive supercoiling arising ahead of the replication forks. We end our review by discussing very recent studies giving a long-sought answer to the question of how slow DNA topoisomerases capable of relaxing just a few positive supercoils per second can counteract the introduction of hundreds of positive supercoils per second ahead of advancing replication forks.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Watson J.D., Crick F.H.C.. Genetical implications of the structure of deoxyribonucleic acids. Nature. 1953; 171:964–967. - PubMed
-
- Bates A.D., Maxwell A.. DNA Topology. 2005; Oxford: Oxford University Press.
-
- Watson J.D., Crick F.H.C.. Molecular structure of nucleic acids. Nature. 1953; 161:737–738. - PubMed
-
- Herbert A., Rich A.. The biology of left-handed Z-DNA. J. Biol. Chem. 1996; 271:11595–11598. - PubMed

