β-Amyloid aggregation and heterogeneous nucleation
- PMID: 31276610
- PMCID: PMC6699094
- DOI: 10.1002/pro.3674
β-Amyloid aggregation and heterogeneous nucleation
Abstract
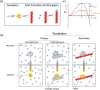
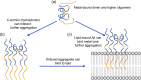
In this article, we consider the role of heterogeneous nucleation in β-amyloid aggregation. Heterogeneous nucleation is more common and occurs at lower levels of supersaturation than homogeneous nucleation. The nucleation period is also the stage at which most of the polymorphism of amyloids arises, this being one of the defining features of amyloids. We focus on several well-known heterogeneous nucleators of β-amyloid, including lipid surfaces, especially those enriched in gangliosides and cholesterol, and divalent metal ions. These two broad classes of nucleators affect β-amyloid particularly in light of the amphiphilicity of these peptides: the N-terminal region, which is largely polar and charged, contains the metal binding site, whereas the C-terminal region is aliphatic and is important in lipid binding. Notably, these two classes of nucleators can interact cooperatively, aggregation begetting greater aggregation.
Keywords: Alzheimer disease; amyloid-β (Aβ) aggregation; cholesterol; gangliosides; heterogeneous nucleation; lipids; metal ions.
© 2019 The Protein Society.
Figures


References
-
- Bender ML, Kezdy J. Mechanism of action of proteolytic enzymes. Annu Rev Biochem. 1965;34:49–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

