Prophylactic Fentanyl Sublingual Spray for Episodic Exertional Dyspnea in Cancer Patients: A Pilot Double-Blind Randomized Controlled Trial
- PMID: 31276809
- PMCID: PMC6754768
- DOI: 10.1016/j.jpainsymman.2019.06.024
Prophylactic Fentanyl Sublingual Spray for Episodic Exertional Dyspnea in Cancer Patients: A Pilot Double-Blind Randomized Controlled Trial
Abstract
Context: The optimal dose of fentanyl sublingual spray (FSS) for exertional dyspnea has not been determined.
Objectives: We examined the effect of two doses of prophylactic FSS on exertional dyspnea.
Methods: In this parallel, dose-finding, double-blind randomized clinical trial, opioid-tolerant cancer patients completed a shuttle walk test at baseline. Patients completed a second shuttle walk test 10 minutes after a single dose of FSS equivalent to either 35%-45% (high dose) or 15%-25% (low dose) of the total daily opioid dose. The primary outcome was change in modified dyspnea Borg scale (0-10) between the first and second shuttle walk tests. Secondary outcomes included adverse events as well as changes in walk distance, vital signs, and neurocognitive function.
Results: Thirty of the 50 enrolled patients completed the study. High-dose FSS (n = 13) resulted in significantly lower dyspnea (mean change -1.42; 95% CI -2.37, -0.48; P = 0.007) and greater walk distance (mean change 44 m; P = 0.001) compared to baseline. Low-dose FSS (n = 17) resulted in a nonsignificant reduction in dyspnea (mean change -0.47; 95% CI -1.26, 0.32; P = 0.24) and significant increase in walk distance (mean change 24 m; P = 0.01) compared to baseline. Global evaluation showed high-dose group was more likely to report at least somewhat better improvement (64% vs. 24%; P = 0.06). No significant adverse events or detriment to vital signs or neurocognitive function was detected.
Conclusion: Prophylactic FSS was well tolerated and demonstrated a dose-response relationship in improving both dyspnea and walk distance. High-dose FSS should be tested in confirmatory trials.
Keywords: Dyspnea; fentanyl; neoplasms; opioid analgesics; physical exertion; randomized controlled trial.
Copyright © 2019 American Academy of Hospice and Palliative Medicine. Published by Elsevier Inc. All rights reserved.
Figures

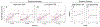
Comment in
-
Authors' Response to: Prophylactic Fentanyl Sublingual Spray for Episodic Exertional Dyspnea in Cancer Patients: A Pilot Double-Blind Randomized Controlled Trial.J Pain Symptom Manage. 2019 Oct;58(4):e17-e18. doi: 10.1016/j.jpainsymman.2019.07.019. Epub 2019 Jul 26. J Pain Symptom Manage. 2019. PMID: 31356961 No abstract available.
-
Response to "Prophylactic Fentanyl Sublingual Spray for Episodic Exertional Dyspnea in Cancer Patients: A Pilot Double-Blind Randomized Controlled Trial".J Pain Symptom Manage. 2019 Oct;58(4):e16-e17. doi: 10.1016/j.jpainsymman.2019.07.020. Epub 2019 Jul 26. J Pain Symptom Manage. 2019. PMID: 31356962 No abstract available.
References
-
- Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med 2009;12:29–36. - PubMed
-
- Simon ST, Weingartner V, Higginson IJ, Voltz R, Bausewein C. Definition, categorization, and terminology of episodic breathlessness: consensus by an international Delphi survey. J Pain Symptom Manage 2014;47:828–838. - PubMed
-
- Simon ST, Bausewein C, Schildmann E, et al. Episodic breathlessness in patients with advanced disease: a systematic review. J Pain Symptom Manage 2013;45:561–578. - PubMed
-
- Mercadante S, Aielli F, Adile C, et al. Epidemiology and Characteristics of Episodic Breathlessness in Advanced Cancer Patients: An Observational Study. J Pain Symptom Manage 2016;51:17–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

