Extracellular vesicle-mediated macrophage activation: An insight into the mechanism of thioredoxin-mediated immune activation
- PMID: 31276937
- PMCID: PMC6612011
- DOI: 10.1016/j.redox.2019.101237
Extracellular vesicle-mediated macrophage activation: An insight into the mechanism of thioredoxin-mediated immune activation
Abstract
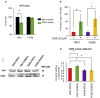
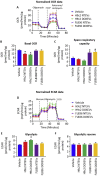
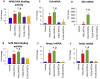
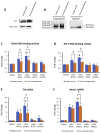
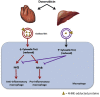
Extracellular vesicles (EVs) generated from redox active anticancer drugs are released into the extracellular environment. These EVs contain oxidized molecules and trigger inflammatory responses by macrophages. Using a mouse model of doxorubicin (DOX)-induced tissue injury, we previously found that the major sources of circulating EVs are from heart and liver, organs that are differentially affected by DOX. Here, we investigated the effects of EVs from cardiomyocytes and those from hepatocytes on macrophage activation. EVs from H9c2 rat cardiomyocytes (H9c2 EVs) and EVs from FL83b mouse hepatocytes (FL83 b EVs) have different levels of protein-bound 4-hydroxynonenal and thus different immunostimulatory effects on mouse RAW264.7 macrophages. H9c2 EVs but not FL83 b EVs induced both pro-inflammatory and anti-inflammatory macrophage activation, mediated by NFκB and Nrf-2 pathways, respectively. DOX enhanced the effects of H9c2 EVs but not FL83 b EVs. While EVs from DOX-treated H9c2 cells (H9c2 DOXEVs) suppressed mitochondrial respiration and increased glycolysis of macrophages, EVs from DOX-treated FL83b cells (FL83b DOXEVs) enhanced mitochondrial reserve capacity. Mechanistically, the different immunostimulatory functions of H9c2 EVs and FL83 b EVs are regulated, in part, by the redox status of the cytoplasmic thioredoxin 1 (Trx1) of macrophages. H9c2 DOXEVs lowered the level of reduced Trx1 in cytoplasm while FL83b DOXEVs did the opposite. Trx1 overexpression alleviated the effect of H9c2 DOXEVs on NFκB and Nrf-2 activation and prevented the upregulation of their target genes. Our findings identify EVs as a novel Trx1-mediated redox mediator of immune response, which greatly enhances our understanding of innate immune responses during cancer therapy.
Keywords: Extracellular vesicles; Macrophage activation; NFκB; Nrf-2; Thioredoxin 1.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Loyer X., Vion A.C., Tedgui A., Boulanger C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014;114:345–353. - PubMed
-
- Fujita Y., Kosaka N., Araya J., Kuwano K., Ochiya T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol. Med. 2015;21:533–542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

