Possible Involvement of Intracellular Calcium-Independent Phospholipase A2 in the Release of Secretory Phospholipases from Mast Cells-Increased Expression in Ileal Mast Cells of Crohn's Disease
- PMID: 31277247
- PMCID: PMC6678282
- DOI: 10.3390/cells8070672
Possible Involvement of Intracellular Calcium-Independent Phospholipase A2 in the Release of Secretory Phospholipases from Mast Cells-Increased Expression in Ileal Mast Cells of Crohn's Disease
Abstract
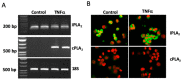
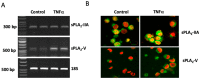
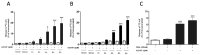
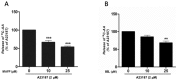
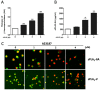
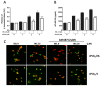
Increased activity of secretory phospholipases A2 (sPLA2) type-II was previously observed in ileum of Crohn's disease (CD). Our aims were to explore the involvement of calcium-independent (i)PLA2β in the release of sPLA2s from the human mast cell (MC) line (HMC-1) and investigate expressions of cytosolic (c)PLA2α, iPLA2β, sPLA2-IIA and sPLA2-V in MCs of CD ileum. The release of sPLA2 was investigated in HMC-1 by immunocytochemistry and ELISA. The expression intensities of PLA2s in mucosal MCs, and the proportion of PLA2-positive MCs, were investigated in normal ileum and in ileum from patients with CD by immunohistochemistry. The calcium ionophore-stimulated release of sPLA2-IIA and sPLA2-V from HMC-1 was reduced by the iPLA2-inhibitor bromoenol lactone. All four PLA2s were detectable in mucosal MCs, both in normal ileum and in CD, but the proportion of iPLA2β-containing mucosal MCs and the expression intensity of sPLA2-IIA was increased in CD. Results indicate that iPLA2β is involved in the secretion of sPLA2s from HMC-1, and suggest that iPLA2β-mediated release of sPLA2 from intestinal MCs may contribute to CD pathophysiology. Ex vivo studies on isolated mucosal mast cells are however needed to clarify the precise role of MC PLA2s in the inflammatory processes of CD.
Keywords: Crohn’s disease; inflammation; mast cells; phospholipases A2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Secreted phospholipase A2 and mast cells.Allergol Int. 2015 Jan;64(1):4-10. doi: 10.1016/j.alit.2014.07.005. Epub 2014 Oct 28. Allergol Int. 2015. PMID: 25572553 Review.
-
Cross-talk between cytosolic phospholipase A2 alpha (cPLA2 alpha) and secretory phospholipase A2 (sPLA2) in hydrogen peroxide-induced arachidonic acid release in murine mesangial cells: sPLA2 regulates cPLA2 alpha activity that is responsible for arachidonic acid release.J Biol Chem. 2003 Jun 27;278(26):24153-63. doi: 10.1074/jbc.M300424200. Epub 2003 Apr 3. J Biol Chem. 2003. PMID: 12676927
-
The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2.J Biol Chem. 1998 Jun 5;273(23):14411-23. doi: 10.1074/jbc.273.23.14411. J Biol Chem. 1998. PMID: 9603953
-
Presence of group IIa secretory phospholipase A2 in mast cells and macrophages in normal human ileal submucosa and in Crohn's disease.Clin Chem Lab Med. 2000 Dec;38(12):1231-6. doi: 10.1515/CCLM.2000.194. Clin Chem Lab Med. 2000. PMID: 11205686
-
Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases.Curr Mol Med. 2001 Dec;1(6):739-54. doi: 10.2174/1566524013363258. Curr Mol Med. 2001. PMID: 11899260 Review.
References
-
- Bischoff S., Gebhardt T. Role of mast cells and eosinophils in neuroimmune interactions regulating mucosal inflammation in inflammatory bowel disease. Adv. Exp. Med. Biol. 2006;579:177–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

