Role of NS1 and TLR3 in Pathogenesis and Immunity of WNV
- PMID: 31277274
- PMCID: PMC6669597
- DOI: 10.3390/v11070603
Role of NS1 and TLR3 in Pathogenesis and Immunity of WNV
Abstract
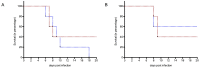
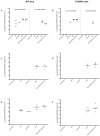
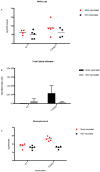
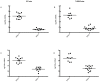
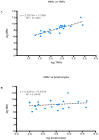
West Nile Virus (WNV) is a mosquito-transmitted flavivirus which causes encephalitis especially in elderly and immunocompromised individuals. Previous studies have suggested the protective role of the Toll-like receptor 3 (TLR3) pathway against WNV entry into the brain, while the WNV non-structural protein 1 (NS1) interferes with the TLR3 signaling pathway, besides being a component of viral genome replication machinery. In this study, we investigated whether immunization with NS1 could protect against WNV neuroinvasion in the context of TLR3 deficiency. We immunized mice with either an intact or deleted TLR3 system (TLR3KO) with WNV envelope glycoprotein (gE) protein, NS1, or a combination of gE and NS1. Immunization with gE or gE/NS1, but not with NS1 alone, induced WNV neutralizing antibodies and protected against WNV brain invasion and inflammation. The presence of intact TLR3 signaling had no apparent effect on WNV brain invasion. However, mock-immunized TLR3KO mice had higher inflammatory cell invasion upon WNV brain infection than NS1-immunized TLR3KO mice and wild type mice. Thus, immunization against NS1 may reduce brain inflammation in a context of TLR3 signaling deficiency.
Keywords: NS1; TLR3; West Nile Virus; West Nile neuroinvasive disease; immunity; pathogenesis; subunit vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Replication-Defective West Nile Virus with NS1 Deletion as a New Vaccine Platform for Flavivirus.J Virol. 2019 Aug 13;93(17):e00720-19. doi: 10.1128/JVI.00720-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31189715 Free PMC article.
-
Levels of Circulating NS1 Impact West Nile Virus Spread to the Brain.J Virol. 2021 Sep 27;95(20):e0084421. doi: 10.1128/JVI.00844-21. Epub 2021 Aug 4. J Virol. 2021. PMID: 34346770 Free PMC article.
-
Human Monoclonal Antibodies against NS1 Protein Protect against Lethal West Nile Virus Infection.mBio. 2021 Oct 26;12(5):e0244021. doi: 10.1128/mBio.02440-21. Epub 2021 Oct 12. mBio. 2021. PMID: 34634945 Free PMC article.
-
The structural immunology of antibody protection against West Nile virus.Immunol Rev. 2008 Oct;225:212-25. doi: 10.1111/j.1600-065X.2008.00676.x. Immunol Rev. 2008. PMID: 18837784 Free PMC article. Review.
-
West Nile virus: immunity and pathogenesis.Viruses. 2011 Jun;3(6):811-28. doi: 10.3390/v3060811. Epub 2011 Jun 15. Viruses. 2011. PMID: 21994755 Free PMC article. Review.
Cited by
-
Post-fever retinitis - Newer concepts.Indian J Ophthalmol. 2020 Sep;68(9):1775-1786. doi: 10.4103/ijo.IJO_1352_20. Indian J Ophthalmol. 2020. PMID: 32823394 Free PMC article. Review.
-
Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses.J Zhejiang Univ Sci B. 2021 Aug 15;22(8):609-632. doi: 10.1631/jzus.B2000808. J Zhejiang Univ Sci B. 2021. PMID: 34414698 Free PMC article. Review.
-
Inflammatory Response Associated with West Nile Neuroinvasive Disease: A Systematic Review.Viruses. 2024 Feb 29;16(3):383. doi: 10.3390/v16030383. Viruses. 2024. PMID: 38543749 Free PMC article.
-
Arthropod-borne encephalitis: an overview for the clinician and emerging considerations.Postgrad Med J. 2023 Jul 21;99(1174):826-833. doi: 10.1136/pmj-2022-142002. Postgrad Med J. 2023. PMID: 37130817 Free PMC article. Review.
-
The Role of Microglia during West Nile Virus Infection of the Central Nervous System.Vaccines (Basel). 2020 Aug 28;8(3):485. doi: 10.3390/vaccines8030485. Vaccines (Basel). 2020. PMID: 32872152 Free PMC article. Review.
References
-
- Sotelo E., Gutierrez-Guzmán A., Del Amo J., Llorente F., El-Harrak M., Pérez-Ramírez E., Blanco J., Höfle U., Jiménez-Clavero M. Pathogenicity of two recent Western Mediterranean West Nile virus isolates in a wild bird species indigenous to Southern Europe: The red-legged partridge. Vet. Res. 2011;42:11. doi: 10.1186/1297-9716-42-11. - DOI - PMC - PubMed
-
- Del Amo J., Llorente F., Figuerola J., Soriguer R.C., Moreno A.M., Cordioli P., Weissenböck H., Jiménez-Clavero M.Á. Experimental infection of house sparrows (Passer domesticus) with West Nile virus isolates of Euro-Mediterranean and North American origins. Vet. Res. 2014;45:33. doi: 10.1186/1297-9716-45-33. - DOI - PMC - PubMed
-
- Pérez-Ramírez E., Llorente F., del Amo J., Nowotny N., Jiménez-Clavero M.Á. Susceptibility and role as competent host of the red-legged partridge after infection with lineage 1 and 2 West Nile virus isolates of Mediterranean and Central European origin. Vet. Microbiol. 2018;222:39–45. doi: 10.1016/j.vetmic.2018.06.012. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

