Application of Porphyrins in Antibacterial Photodynamic Therapy
- PMID: 31277423
- PMCID: PMC6650910
- DOI: 10.3390/molecules24132456
Application of Porphyrins in Antibacterial Photodynamic Therapy
Abstract
Antibiotics are commonly used to control, treat, or prevent bacterial infections, however bacterial resistance to all known classes of traditional antibiotics has greatly increased in the past years especially in hospitals rendering certain therapies ineffective. To limit this emerging public health problem, there is a need to develop non-incursive, non-toxic, and new antimicrobial techniques that act more effectively and quicker than the current antibiotics. One of these effective techniques is antibacterial photodynamic therapy (aPDT). This review focuses on the application of porphyrins in the photo-inactivation of bacteria. Mechanisms of bacterial resistance and some of the current 'greener' methods of synthesis of meso-phenyl porphyrins are discussed. In addition, significance and limitations of aPDT are also discussed. Furthermore, we also elaborate on the current clinical applications and the future perspectives and directions of this non-antibiotic therapeutic strategy in combating infectious diseases.
Keywords: antibacterial photodynamic therapy; bacteria; light; nanoparticles; porphyrins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
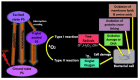
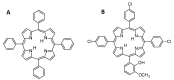
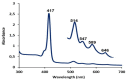


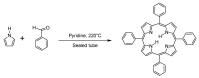









Similar articles
-
Blue-Light-Activated Water-Soluble Sn(IV)-Porphyrins for Antibacterial Photodynamic Therapy (aPDT) against Drug-Resistant Bacterial Pathogens.Mol Pharm. 2024 May 6;21(5):2365-2374. doi: 10.1021/acs.molpharmaceut.3c01162. Epub 2024 Apr 15. Mol Pharm. 2024. PMID: 38620059
-
Photodynamic Chitosan Nano-Assembly as a Potent Alternative Candidate for Combating Antibiotic-Resistant Bacteria.ACS Appl Mater Interfaces. 2019 Jul 31;11(30):26711-26721. doi: 10.1021/acsami.9b09020. Epub 2019 Jul 19. ACS Appl Mater Interfaces. 2019. PMID: 31287648
-
Antimicrobial photodynamic therapy by means of porphycene photosensitizers.J Photochem Photobiol B. 2017 Sep;174:84-89. doi: 10.1016/j.jphotobiol.2017.07.016. Epub 2017 Jul 21. J Photochem Photobiol B. 2017. PMID: 28756156
-
Advancements of Porphyrin-Derived Nanomaterials for Antibacterial Photodynamic Therapy and Biofilm Eradication.Adv Healthc Mater. 2024 Oct;13(27):e2401211. doi: 10.1002/adhm.202401211. Epub 2024 Jul 27. Adv Healthc Mater. 2024. PMID: 39073000 Review.
-
Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria.Eur J Med Chem. 2019 Aug 1;175:72-106. doi: 10.1016/j.ejmech.2019.04.057. Epub 2019 Apr 29. Eur J Med Chem. 2019. PMID: 31096157 Review.
Cited by
-
Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin.J Fungi (Basel). 2022 May 25;8(6):556. doi: 10.3390/jof8060556. J Fungi (Basel). 2022. PMID: 35736039 Free PMC article.
-
Simple, Axial Ligand-Mediated Route to Water-Soluble Iridium Corroles.ACS Omega. 2021 Jun 15;6(25):16683-16687. doi: 10.1021/acsomega.1c02399. eCollection 2021 Jun 29. ACS Omega. 2021. PMID: 34235340 Free PMC article.
-
Photoinactivation of Pseudomonas aeruginosa Biofilm by Dicationic Diaryl-Porphyrin.Int J Mol Sci. 2021 Jun 24;22(13):6808. doi: 10.3390/ijms22136808. Int J Mol Sci. 2021. PMID: 34202773 Free PMC article.
-
Recent advances in NIR-II photothermal and photodynamic therapies for drug-resistant wound infections.Mater Today Bio. 2025 May 14;32:101871. doi: 10.1016/j.mtbio.2025.101871. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40496729 Free PMC article.
-
Combatting Antibiotic Resistance Using Supramolecular Assemblies.Pharmaceuticals (Basel). 2022 Jun 28;15(7):804. doi: 10.3390/ph15070804. Pharmaceuticals (Basel). 2022. PMID: 35890105 Free PMC article. Review.
References
-
- Almeida A., Cunha A., Faustino M.A.F., Tomé A.C., Neves M.G.P.M.S. Porphyrins as Antimicrobial Photosensitizing Agents. In: Hamblin M.R., Jori G., editors. Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications. Volume 11. RSC Publishing; Cambridge, UK: 2011. pp. 83–160.
-
- Maisch T., Hackbarth S., Regensburger J., Felgenträger A., Bäumler W., Landthaler M., Röder B. Photodynamic inactivation of multi resistant bacteria (PIB)–a new approach to treat superficial infections in the 21st century. J. Dtsch. Dermatologischen Ges. 2011;9:360–366. doi: 10.1111/j.1610-0387.2010.07577.x. - DOI - PubMed
-
- Pollack A. Rising Threat of Infections Unfazed by Antibiotics, New York Times. [(accessed on 11 November 2018)]; Available online: http://www.biocence.com/download/raging_antibiotic.pdf.
-
- Songca S.P., Oluwafemi O.S. Photodynamic therapy: A new light for the developing world. Afr. J. Biotechnol. 2013;12:3590–3599. doi: 10.5897/AJB12.2586. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

