EDB-FN Targeted Peptide-Drug Conjugates for Use against Prostate Cancer
- PMID: 31277465
- PMCID: PMC6651341
- DOI: 10.3390/ijms20133291
EDB-FN Targeted Peptide-Drug Conjugates for Use against Prostate Cancer
Abstract
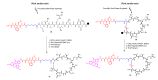
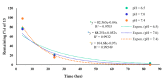
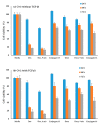
Prostate cancer (PCa) is the most common malignancy in men and is the leading cause of cancer-related male mortality. A disulfide cyclic peptide ligand [CTVRTSADC] 1 has been previously found to target extra domain B of fibronectin (EDB-FN) in the extracellular matrix that can differentiate aggressive PCa from benign prostatic hyperplasia. We synthesized and optimized the stability of ligand 1 by amide cyclization to obtain [KTVRTSADE] 8 using Fmoc/tBu solid-phase chemistry. Optimized targeting ligand 8 was found to be stable in phosphate buffered saline (PBS, pH 6.5, 7.0, and 7.5) and under redox conditions, with a half-life longer than 8 h. Confocal microscopy studies demonstrated increased binding of ligand 8 to EDB-FN compared to ligand 1. Therefore, we hypothesized that the EDB-FN targeted peptides (1 and 8) conjugated with an anticancer drug via a hydrolyzable linker would provide selective cytotoxicity to the cancer cells. To test our hypothesis, we selected both the normal prostate cell line, RWPE-1, and the cancerous prostate cell lines, PC3, DU-145, LNCaP, and C4-2, to evaluate the anticancer activity of synthesized peptide-drug conjugates. Docetaxel (Doce) and doxorubicin (Dox) were used as anticancer drugs. Dox conjugate 13 containing disulfide linkage showed comparable cytotoxicity versus Dox after 72 h incubation in all the cancer cell lines, whereas it was found to be less cytotoxic on RWPE-1, suggesting that it can act as a Dox prodrug. Doce conjugate 14 was found to be less cytotoxic in all the cell lines as compared to drug alone.
Keywords: Fmoc/tBu; antiproliferative assay; conjugation; docetaxel; doxorubicin; extra domain B; fibronectin; peptide–drug conjugate; prostate cancer; solid-phase synthesis; targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
-
- American Cancer Society Prostate Cancer. [(accessed on 16 February 2018)]; Available online: https://www.cancer.org/cancer/prostate-cancer.html.
-
- Summers N., Vanderpuye-Orgle J., Reinhart M., Gallagher M., Sartor O. Efficacy and safety of post-docetaxel therapies in metastatic castration-resistant prostate cancer: a systematic review of the literature. Curr. Med. Res. Opin. 2017;33:1995–2008. doi: 10.1080/03007995.2017.1341869. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

