Gene Expression Comparison between the Lymph Node-Positive and -Negative Reveals a Peculiar Immune Microenvironment Signature and a Theranostic Role for WNT Targeting in Pancreatic Ductal Adenocarcinoma: A Pilot Study
- PMID: 31277479
- PMCID: PMC6678707
- DOI: 10.3390/cancers11070942
Gene Expression Comparison between the Lymph Node-Positive and -Negative Reveals a Peculiar Immune Microenvironment Signature and a Theranostic Role for WNT Targeting in Pancreatic Ductal Adenocarcinoma: A Pilot Study
Abstract
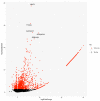
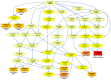
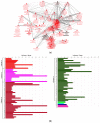
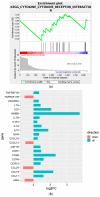
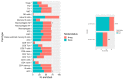
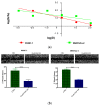
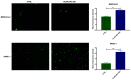
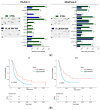
Over the past several years there has been much debate with regards to the prognostic and clinical significance of pancreatic ductal adenocarcinoma (PDAC) with lymph nodes metastasis. The PDAC gene expression knowledge and the biologic alterations underlying the lymph node involvement convey a clinical implication in dealing with the theranostic window. To this end, we provide an original bioinformatic dissection of the gene expression differences of PDAC according to the nodal involvement from a large public available dataset. Comprehensive transcriptomic analysis from 143 RNA-seq patient's derived samples indicated that WNT increased activation and a peculiar immune microenvironment identify subjects with nodal involvement. In frame of this thinking, we validated the WNT pathway role in increasing the likelihood of lymphatic dissemination in vitro. Moreover, we demonstrated for the first time in a PDAC model the potential therapeutic window that XAV-939-a specific WNT pathway inhibitor-has in re-educating a tumor-permissive immune system. Finally, we outline the potential implication on bystander molecular drivers exerted by WNT molecular inhibition, providing a picture of the proteomic oncogenic landscape changes elicited by XAV-939 on PDAC cells and their clinical implication. Our findings hold the promise to identify novel immune-based therapeutic strategies targeting WNT to enhance PDAC cytotoxicity and restore anti-PDAC immunity in node-positive disease.
Keywords: ANGPTL4; M2 macrophages; MST-1; PDAC; WNT; XAV-939; dendritic cells; lymph node metastases; pancreatic cancer; tumor immune microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- National Comprehensive Cancer Network NCCN Guidelines Version 2.2019 Pancreatic Adenocarcinoma. [(accessed on 5 May 2019)]; Available online: http://www.nccn.org/patients.
-
- Khorana A.A., Mangu P.B., Berlin J., Engebretson A., Hong T.S., Maitra A., Mohile S.G., Mumber M., Schulick R., Shapiro M., et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017;35:2324–2328. doi: 10.1200/JCO.2017.72.4948. - DOI - PubMed
-
- Isaji S., Mizuno S., Windsor J.A., Bassi C., Fernández-del Castillo C., Hackert T., Hayasaky A., Katz M.H.G., Kim S.W., Kishiwada M., et al. International consensus on definition and criteria of borderline resecTable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. doi: 10.1016/j.pan.2017.11.011. - DOI - PubMed
-
- Kaissis G.A., Lohöfer F.K., Ziegelmayer S., Danner J., Jäger C., Schirren R., Ankerst D., Ceyhan G.O., Friess H., Rummeny E.J., et al. Borderline-resectable pancreatic adenocarcinoma: Contour irregularity of the venous confluence in pre-operative computed tomography predicts histopathological infiltration. PLoS ONE. 2019;14:e0208717. doi: 10.1371/journal.pone.0208717. - DOI - PMC - PubMed
-
- Silvestris N., Brunetti O., Vasile E., Cellini F., Cataldo I., Pusceddu V., Cattaneo M., Partelli S., Scartozzi M., Aprile G., et al. Multimodal treatment of resectable pancreatic ductal adenocarcinoma. Crit. Rev. Oncol. Hematol. 2017;111:152–165. doi: 10.1016/j.critrevonc.2017.01.015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

