Natural and Drug Rewards Engage Distinct Pathways that Converge on Coordinated Hypothalamic and Reward Circuits
- PMID: 31277924
- PMCID: PMC6728176
- DOI: 10.1016/j.neuron.2019.05.050
Natural and Drug Rewards Engage Distinct Pathways that Converge on Coordinated Hypothalamic and Reward Circuits
Abstract
Motivated behavior is influenced by neural networks that integrate physiological needs. Here, we describe coordinated regulation of hypothalamic feeding and midbrain reward circuits in awake behaving mice. We find that alcohol and other non-nutritive drugs inhibit activity in hypothalamic feeding neurons. Interestingly, nutrients and drugs utilize different pathways for the inhibition of hypothalamic neuron activity, as alcohol signals hypothalamic neurons in a vagal-independent manner, while fat and satiation signals require the vagus nerve. Concomitantly, nutrients, alcohol, and drugs also increase midbrain dopamine signaling. We provide evidence that these changes are interdependent, as modulation of either hypothalamic neurons or midbrain dopamine signaling influences reward-evoked activity changes in the other population. Taken together, our results demonstrate that (1) food and drugs can engage at least two peripheral→central pathways to influence hypothalamic neuron activity, and (2) hypothalamic and dopamine circuits interact in response to rewards.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures

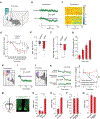

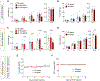
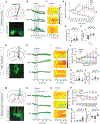



Comment in
-
A Circuit Perspective on State-Dependent Effects of Dopamine Stimulants.Neuron. 2019 Sep 4;103(5):755-756. doi: 10.1016/j.neuron.2019.08.022. Neuron. 2019. PMID: 31487524
References
-
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderon NC, Esposti F, Borghuis BG, Sun XR, et al. (2012). Optimization of a GCaMP calcium indicator for neural activity imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 13819–13840. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

