Intestinal lipid droplets as novel mediators of host-pathogen interaction in Drosophila
- PMID: 31278163
- PMCID: PMC6679391
- DOI: 10.1242/bio.039040
Intestinal lipid droplets as novel mediators of host-pathogen interaction in Drosophila
Abstract
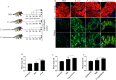
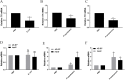
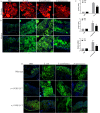
Lipid droplets (LDs) are lipid-carrying multifunctional organelles, which might also interact with pathogens and influence the host immune response. However, the exact nature of these interactions remains currently unexplored. Here we show that systemic infection of Drosophila adult flies with non-pathogenic Escherichia coli, the extracellular bacterial pathogen Photorhabdus luminescens or the facultative intracellular pathogen Photorhabdus asymbiotica results in intestinal steatosis marked by lipid accumulation in the midgut. Accumulation of LDs in the midgut also correlates with increased whole-body lipid levels characterized by increased expression of genes regulating lipogenesis. The lipid-enriched midgut further displays reduced expression of the enteroendocrine-secreted hormone, Tachykinin. The observed lipid accumulation requires the Gram-negative cell wall pattern recognition molecule, PGRP-LC, but not PGRP-LE, for the humoral immune response. Altogether, our findings indicate that Drosophila LDs are inducible organelles, which can serve as markers for inflammation and, depending on the nature of the challenge, they can dictate the outcome of the infection.
Keywords: Drosophila; Infection; Lipid droplets; Midgut.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
-
- Araujo-Santos T., Rodriguez N. E., Moura-Pontes S., Dixt U. G., Abanades D. R., Bozza P. T., Wilson M. E. and Borges V. M. (2014). Role of prostaglandin F2alpha production in lipid bodies from Leishmania infantum chagasi: insights on virulence. J. Infect. Dis. 210, 1951-1961. 10.1093/infdis/jiu299 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

