Anionic and zwitterionic moieties as widespread glycan modifications in non-vertebrates
- PMID: 31278613
- PMCID: PMC6994554
- DOI: 10.1007/s10719-019-09874-2
Anionic and zwitterionic moieties as widespread glycan modifications in non-vertebrates
Abstract
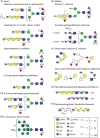
Glycan structures in non-vertebrates are highly variable; it can be assumed that this is a product of evolution and speciation, not that it is just a random event. However, in animals and protists, there is a relatively limited repertoire of around ten monosaccharide building blocks, most of which are neutral in terms of charge. While two monosaccharide types in eukaryotes (hexuronic and sialic acids) are anionic, there are a number of organic or inorganic modifications of glycans such as sulphate, pyruvate, phosphate, phosphorylcholine, phosphoethanolamine and aminoethylphosphonate that also confer a 'charged' nature (either anionic or zwitterionic) to glycoconjugate structures. These alter the physicochemical properties of the glycans to which they are attached, change their ionisation when analysing them by mass spectrometry and result in different interactions with protein receptors. Here, we focus on N-glycans carrying anionic and zwitterionic modifications in protists and invertebrates, but make some reference to O-glycans, glycolipids and glycosaminoglycans which also contain such moieties. The conclusion is that 'charged' glycoconjugates are a widespread, but easily overlooked, feature of 'lower' organisms.
Keywords: Glucuronic acid; Glycans; Glycomics; Insect; Mollusc; Nematode; Phosphoethanolamine; Phosphorylcholine; Sulphate.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

