Liver sympathetic denervation reverses obesity-induced hepatic steatosis
- PMID: 31278754
- PMCID: PMC6716997
- DOI: 10.1113/JP277994
Liver sympathetic denervation reverses obesity-induced hepatic steatosis
Abstract
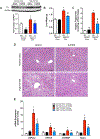
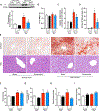
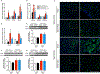
Key points: Non-alcoholic fatty liver disease, characterized in part by elevated liver triglycerides (i.e. hepatic steatosis), is a growing health problem. In this study, we found that hepatic steatosis is associated with robust hepatic sympathetic overactivity. Removal of hepatic sympathetic nerves reduced obesity-induced hepatic steatosis. Liver sympathetic innervation modulated hepatic lipid acquisition pathways during obesity.
Abstract: Non-alcoholic fatty liver disease (NAFLD) affects 1 in 3 Americans and is a significant risk factor for type II diabetes mellitus, insulin resistance and hepatic carcinoma. Characterized in part by excessive hepatic triglyceride accumulation (i.e. hepatic steatosis), the incidence of NAFLD is increasing - in line with the growing obesity epidemic. The role of the autonomic nervous system in NAFLD remains unclear. Here, we show that chronic hepatic sympathetic overactivity mediates hepatic steatosis. Direct multiunit recordings of hepatic sympathetic nerve activity were obtained in high fat diet and normal chow fed male C57BL/6J mice. To reduce hepatic sympathetic nerve activity we utilized two approaches including pharmacological ablation of the sympathetic nerves and phenol-based hepatic sympathetic nerve denervation. Diet-induced NAFLD was associated with a nearly doubled firing rate of the hepatic sympathetic nerves, which was largely due to an increase in efferent nerve traffic. Furthermore, established high fat diet-induced hepatic steatosis was effectively reduced with pharmacological or phenol-based removal of the hepatic sympathetic nerves, independent of changes in body weight, caloric intake or adiposity. Ablation of liver sympathetic nerves was also associated with improvements in liver triglyceride accumulation pathways including free fatty acid uptake and de novo lipogenesis. These findings highlight an unrecognized pathogenic link between liver sympathetic outflow and hepatic steatosis and suggest that manipulation of the liver sympathetic nerves may represent a novel therapeutic strategy for NAFLD.
Keywords: autonomic nervous system; diet-induced obesity; non-alcoholic fatty liver disease; sympathetic nerve activity.
© 2019 The Authors. The Journal of Physiology © 2019 The Physiological Society.
Conflict of interest statement
Competing Interests
None
Figures



Comment in
-
Restoring the autonomic balance to reduce liver steatosis.J Physiol. 2019 Sep;597(18):4683-4684. doi: 10.1113/JP278567. Epub 2019 Aug 21. J Physiol. 2019. PMID: 31393006 Free PMC article. No abstract available.
-
Liver sympathetic nerve activity and steatosis.J Physiol. 2020 Jan;598(1):11-12. doi: 10.1113/JP278895. Epub 2019 Dec 3. J Physiol. 2020. PMID: 31646632 No abstract available.
References
-
- Angulo P (2002). Nonalcoholic fatty liver disease. The New England journal of medicine 346, 1221–1231. - PubMed
-
- Bruinstroop E, Eliveld J, Foppen E, Busker S, Ackermans MT, Fliers E & Kalsbeek A. (2015). Hepatic denervation and dyslipidemia in obese Zucker (fa/fa) rats. Int J Obes (Lond) 39, 1655–1658. - PubMed
-
- Carreno FR & Seelaender MC. (2004). Liver denervation affects hepatocyte mitochondrial fatty acid transport capacity. Cell Biochem Funct 22, 9–17. - PubMed
-
- Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS & Schaffer JE. (2005). Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circulation research 96, 225–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

