Mammalian diaphanous-related formin 1 (mDia1) coordinates mast cell migration and secretion through its actin-nucleating activity
- PMID: 31279009
- PMCID: PMC7278082
- DOI: 10.1016/j.jaci.2019.06.028
Mammalian diaphanous-related formin 1 (mDia1) coordinates mast cell migration and secretion through its actin-nucleating activity
Abstract
Background: Actin remodeling is a key regulator of mast cell (MC) migration and secretion. However, the precise mechanism underlying the coordination of these processes has remained obscure.
Objective: We sought to characterize the actin rearrangements that occur during MC secretion or chemotactic migration and identify the underlying mechanism of their coordination.
Methods: Using high-resolution microscopy, we analyzed the dynamics of actin rearrangements in MCs triggered to migration by IL-8 or prostaglandin E2 or to FcεRI-stimulated secretion.
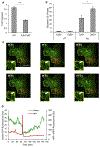
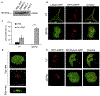
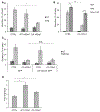
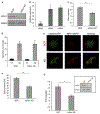
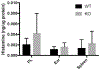
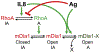
Results: We show that a major feature of the actin skeleton in MCs stimulated to migration is the buildup of pericentral actin clusters that prevent cell flattening and converge the secretory granules (SGs) in the cell center. This migratory phenotype is replaced on encounter of an IgE cross-linking antigen that stimulates secretion through a secretory phenotype characterized by cell flattening, reduction of actin mesh density, ruffling of cortical actin, and mobilization of SGs. Furthermore, we show that knockdown of mammalian diaphanous-related formin 1 (mDia1) inhibits chemotactic migration and its typical actin rearrangements, whereas expression of an active mDia1 mutant recapitulates the migratory actin phenotype and enhances cell migration while inhibiting FcεRI-triggered secretion. However, mice deficient in mDia1 appear to have normal numbers of MCs in various organs at baseline.
Conclusion: Our results demonstrate a unique role of actin rearrangements in clustering the SGs and inhibiting their secretion during MC migration. We identify mDia1 as a novel regulator of MC response that coordinates MC chemotaxis and secretion through its actin-nucleating activity.
Keywords: Mast cells; actin; chemotaxis; exocytosis; mammalian diaphanous-related formin 1.
Copyright © 2019 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Figures



Similar articles
-
The actin cytoskeleton and mast cell function.Curr Opin Immunol. 2021 Oct;72:27-33. doi: 10.1016/j.coi.2021.03.002. Epub 2021 Mar 22. Curr Opin Immunol. 2021. PMID: 33765561 Review.
-
Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways.Circ Res. 2012 May 11;110(10):1279-93. doi: 10.1161/CIRCRESAHA.111.262519. Epub 2012 Apr 17. Circ Res. 2012. PMID: 22511750 Free PMC article.
-
Directing exocrine secretory vesicles to the apical membrane by actin cables generated by the formin mDia1.Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10652-7. doi: 10.1073/pnas.1303796110. Epub 2013 Jun 10. Proc Natl Acad Sci U S A. 2013. PMID: 23754409 Free PMC article.
-
Prevention of F-actin assembly switches the response to SCF from chemotaxis to degranulation in human mast cells.Eur J Immunol. 2013 Jul;43(7):1873-82. doi: 10.1002/eji.201243214. Epub 2013 Jun 4. Eur J Immunol. 2013. PMID: 23616175 Free PMC article.
-
Nucleating amoeboid cancer cell motility with Diaphanous related formins.Cytoskeleton (Hoboken). 2025 Mar;82(3):91-97. doi: 10.1002/cm.21880. Epub 2024 May 18. Cytoskeleton (Hoboken). 2025. PMID: 38761126 Free PMC article. Review.
Cited by
-
Transmembrane formins as active cargoes of membrane trafficking.J Exp Bot. 2024 Jun 24;75(12):3668-3684. doi: 10.1093/jxb/erae078. J Exp Bot. 2024. PMID: 38401146 Free PMC article. Review.
-
β2-Integrins - Regulatory and Executive Bridges in the Signaling Network Controlling Leukocyte Trafficking and Migration.Front Immunol. 2022 Apr 22;13:809590. doi: 10.3389/fimmu.2022.809590. eCollection 2022. Front Immunol. 2022. PMID: 35529883 Free PMC article. Review.
-
DIAPH1-Deficiency is Associated with Major T, NK and ILC Defects in Humans.J Clin Immunol. 2024 Aug 9;44(8):175. doi: 10.1007/s10875-024-01777-8. J Clin Immunol. 2024. PMID: 39120629 Free PMC article.
-
Actin and Myosin in Non-Neuronal Exocytosis.Cells. 2020 Jun 11;9(6):1455. doi: 10.3390/cells9061455. Cells. 2020. PMID: 32545391 Free PMC article. Review.
-
Qingkailing injection induces pseudo-allergic reactions via the MRGPRX2 pathway.Am J Transl Res. 2025 Mar 15;17(3):2178-2187. doi: 10.62347/MFBU4210. eCollection 2025. Am J Transl Res. 2025. PMID: 40226010 Free PMC article.
References
-
- Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol 2014;14:478–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

