Evaluation of an adjuvanted hydrogel-based pDNA nanoparticulate vaccine for rabies prevention and immunocontraception
- PMID: 31279062
- PMCID: PMC11287484
- DOI: 10.1016/j.nano.2019.102049
Evaluation of an adjuvanted hydrogel-based pDNA nanoparticulate vaccine for rabies prevention and immunocontraception
Abstract
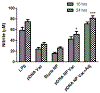
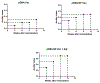
Immunocontraceptive vaccination is becoming an acceptable strategy in managing animal populations. Mass vaccination of dogs is the most cost-effective and efficient method to control rabies, and combination of rabies vaccination and animal population control will be an added advantage. In this study, we developed an adjuvanted hydrogel-based pDNA nanoparticulate vaccine for rabies protection and immunocontraception. In vivo, we observed an immune response skewed toward a Th2 type, in contrast to the Th1 type in our previous pDNA study. The observation was verified by the IgG2a/IgG1 ratio (<1), and cytokine expression profile of IL-4 and IFN-γ. The humoral immune response is key for rabies protection and a GnRH antibody-based immunocontraception. In mice, anti-GnRH antibody titers were detected 4 weeks after immunization and lasted for 12 weeks, post animal experiment was terminated. The adjuvanted pDNA nanoparticulate vaccine shows promise for future studies evaluating protection from rabies challenge and prevention of animal breeding.
Keywords: Gonadotrophin-releasing hormone (GnRH); Immunocontraceptive vaccine; Poloxamer gels; Rabies.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure
The authors report no conflicts of interest in this work. The authors alone are responsible for the content and writing of this article.
Figures



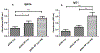




References
-
- Bose A, Munshi R, Tripathy RM, Madhusudana SN, Harish BR, Thaker S, Mahendra BJ, Gunale B, Gogtay NJ, Thatte UM, Mani RS, Manjunath K, George K, Yajaman AB, Sahai A, Dhere RM, Alex RG, Adhikari DD, Abhilash null, Raghava V, Kumbhar D, Behera TR, Kulkarni PS, A randomized non-inferiority clinical study to assess post-exposure prophylaxis by a new purified vero cell rabies vaccine (Rabivax-S) administered by intramuscular and intradermal routes, Vaccine. 34 (2016) 4820–4826. doi: 10.1016/j.vaccine.2016.08.005. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

