Interaction of blood plasma proteins with superhemophobic titania nanotube surfaces
- PMID: 31279063
- PMCID: PMC6814547
- DOI: 10.1016/j.nano.2019.102046
Interaction of blood plasma proteins with superhemophobic titania nanotube surfaces
Abstract
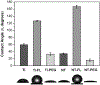
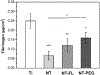
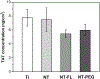
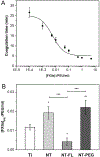
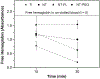
The need to improve blood biocompatibility of medical devices is urgent. As soon as blood encounters a biomaterial implant, proteins adsorb on its surfaces, often leading to several complications such as thrombosis and failure of the device. Therefore, controlling protein adsorption plays a major role in developing hemocompatible materials. In this study, the interaction of key blood plasma proteins with superhemophobic titania nanotube substrates and the blood clotting responses was investigated. The substrate stability was evaluated and fibrinogen adsorption and thrombin formation from plasma were assessed using ELISA. Whole blood clotting kinetics was also investigated, and Factor XII activation on the substrates was characterized by an in vitro plasma coagulation time assay. The results show that superhemophobic titania nanotubes are stable and considerably decrease surface protein adsorption/Factor XII activation as well as delay the whole blood clotting, and thus can be a promising approach for designing blood contacting medical devices.
Keywords: Factor XII; Fibrinogen; Hemocompatibility; Protein adsorption; Superhemophobic; Titania nanotubes.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

