The multifaceted role of TMEM16A in cancer
- PMID: 31279157
- PMCID: PMC6711484
- DOI: 10.1016/j.ceca.2019.06.004
The multifaceted role of TMEM16A in cancer
Abstract
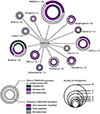
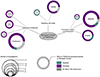
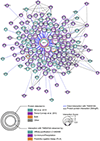
The calcium-activated chloride channel TMEM16A is intimately linked to cancers. Over decades, TMEM16A over-expression and contribution to prognosis have been widely studied for multiple cancers strengthening the idea that TMEM16A could be a valuable biomarker and a promising therapeutic target. Surprisingly, from the survey of the literature, it appears that TMEM16A has been involved in multiple cancer-related functions and a large number of molecular targets of TMEM16A have been proposed. Thus, TMEM16A appears to be an ion channel with a multifaceted role in cancers. In this review, we summarize the latest development regarding TMEM16A contribution to cancers. We will survey TMEM16A contribution in cancer prognosis, the origins of its over-expression in cancer cells, the multiple biological functions and molecular pathways regulated by TMEM16A. Then, we will consider the question regarding the molecular mechanism of TMEM16A in cancers and the possible basis for the multifaceted role of TMEM16A in cancers.
Keywords: Anoctamin-1; Calcium-activated chloride channels; Cancer; TMEM16A.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest:
No conflict of interest to declare
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

