In Vivo Outcome of Homology-Directed Repair at the HBB Gene in HSC Using Alternative Donor Template Delivery Methods
- PMID: 31279229
- PMCID: PMC6611979
- DOI: 10.1016/j.omtn.2019.05.025
In Vivo Outcome of Homology-Directed Repair at the HBB Gene in HSC Using Alternative Donor Template Delivery Methods
Abstract
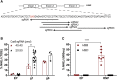
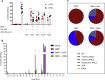
Gene editing following designer nuclease cleavage in the presence of a DNA donor template can revert mutations in disease-causing genes. For optimal benefit, reversion of the point mutation in HBB leading to sickle cell disease (SCD) would permit precise homology-directed repair (HDR) while concurrently limiting on-target non-homologous end joining (NHEJ)-based HBB disruption. In this study, we directly compared the relative efficiency of co-delivery of a novel CRISPR/Cas9 ribonucleoprotein targeting HBB in association with recombinant adeno-associated virus 6 (rAAV6) versus single-stranded oligodeoxynucleotides (ssODNs) to introduce the sickle mutation (GTC or GTG; encoding E6V) or a silent change (GAA; encoding E6optE) in human CD34+ mobilized peripheral blood stem cells (mPBSCs) derived from healthy donors. In vitro, rAAV6 outperformed ssODN donor template delivery and mediated greater HDR correction, leading to both higher HDR rates and a higher HDR:NHEJ ratio. In contrast, at 12-14 weeks post-transplant into recipient, immunodeficient, NOD, B6, SCID Il2rγ-/- Kit(W41/W41) (NBSGW) mice, a ∼6-fold higher proportion of ssODN-modified cells persisted in vivo compared to recipients of rAAV6-modified mPBSCs. Together, our findings highlight that methodology for donor template delivery markedly impacts long-term persistence of HBB gene-modified mPBSCs, and they suggest that the ssODN platform is likely to be most amenable to direct clinical translation.
Keywords: CD34; Crispr/Cas9; NBSGW41 mice; NHEJ versus HDR; gene editing; hematopoietic stem cells; hemoglobin disorders; homology-directed repair; in vivo engraftment; rAAV6; sickle cell disease; ssODN; stem cell cures.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Editing the Sickle Cell Disease Mutation in Human Hematopoietic Stem Cells: Comparison of Endonucleases and Homologous Donor Templates.Mol Ther. 2019 Aug 7;27(8):1389-1406. doi: 10.1016/j.ymthe.2019.05.014. Epub 2019 May 24. Mol Ther. 2019. PMID: 31178391 Free PMC article.
-
Gene correction of HBB mutations in CD34+ hematopoietic stem cells using Cas9 mRNA and ssODN donors.Mol Cell Pediatr. 2018 Nov 14;5(1):9. doi: 10.1186/s40348-018-0086-1. Mol Cell Pediatr. 2018. PMID: 30430274 Free PMC article.
-
Efficient Generation of Gene-Modified Pigs Harboring Precise Orthologous Human Mutation via CRISPR/Cas9-Induced Homology-Directed Repair in Zygotes.Hum Mutat. 2016 Jan;37(1):110-8. doi: 10.1002/humu.22913. Epub 2015 Oct 23. Hum Mutat. 2016. PMID: 26442986
-
Advance trends in targeting homology-directed repair for accurate gene editing: An inclusive review of small molecules and modified CRISPR-Cas9 systems.Bioimpacts. 2022;12(4):371-391. doi: 10.34172/bi.2022.23871. Epub 2022 Jun 22. Bioimpacts. 2022. PMID: 35975201 Free PMC article. Review.
-
Methods Favoring Homology-Directed Repair Choice in Response to CRISPR/Cas9 Induced-Double Strand Breaks.Int J Mol Sci. 2020 Sep 4;21(18):6461. doi: 10.3390/ijms21186461. Int J Mol Sci. 2020. PMID: 32899704 Free PMC article. Review.
Cited by
-
Choice of template delivery mitigates the genotoxic risk and adverse impact of editing in human hematopoietic stem cells.Cell Stem Cell. 2022 Oct 6;29(10):1428-1444.e9. doi: 10.1016/j.stem.2022.09.001. Cell Stem Cell. 2022. PMID: 36206730 Free PMC article.
-
CRISPR Editing Enables Consequential Tag-Activated MicroRNA-Mediated Endogene Deactivation.Int J Mol Sci. 2022 Jan 19;23(3):1082. doi: 10.3390/ijms23031082. Int J Mol Sci. 2022. PMID: 35163006 Free PMC article.
-
Gene Editing-Based Technologies for Beta-hemoglobinopathies Treatment.Biology (Basel). 2022 Jun 4;11(6):862. doi: 10.3390/biology11060862. Biology (Basel). 2022. PMID: 35741383 Free PMC article. Review.
-
High-level correction of the sickle mutation is amplified in vivo during erythroid differentiation.iScience. 2022 May 10;25(6):104374. doi: 10.1016/j.isci.2022.104374. eCollection 2022 Jun 17. iScience. 2022. PMID: 35633935 Free PMC article.
-
Cas9 protein delivery non-integrating lentiviral vectors for gene correction in sickle cell disease.Mol Ther Methods Clin Dev. 2021 Mar 3;21:121-132. doi: 10.1016/j.omtm.2021.02.022. eCollection 2021 Jun 11. Mol Ther Methods Clin Dev. 2021. PMID: 33816645 Free PMC article.
References
-
- Kato G.J., Piel F.B., Reid C.D., Gaston M.H., Ohene-Frempong K., Krishnamurti L., Smith W.R., Panepinto J.A., Weatherall D.J., Costa F.F., Vichinsky E.P. Sickle cell disease. Nat. Rev. Dis. Primers. 2018;4:18010. - PubMed
-
- Piel F.B., Steinberg M.H., Rees D.C. Sickle Cell Disease. N. Engl. J. Med. 2017;376:1561–1573. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

