Cryopreservation in 95% serum with 5% DMSO maintains colony formation and chondrogenic abilities in human synovial mesenchymal stem cells
- PMID: 31279341
- PMCID: PMC6612159
- DOI: 10.1186/s12891-019-2700-3
Cryopreservation in 95% serum with 5% DMSO maintains colony formation and chondrogenic abilities in human synovial mesenchymal stem cells
Abstract
Background: Synovial mesenchymal stem cells (MSCs) are an attractive cell source for cartilage and meniscus regeneration. The optimum cryopreservation medium has not been determined, but dimethylsulfoxide (DMSO) should be excluded, if possible, because of its toxicity. The purposes of our study were to examine the possible benefits of higher concentrations of serum and the effectiveness of 100% serum (without DMSO) for the cryopreservation of synovial MSCs.
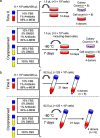
Methods: Human synovium was harvested from the knees of four donors with osteoarthritis during total knee arthroplasty. Synovial MSCs (8 × 105 cells) were suspended in 400 μL medium and used as a Time 0 control. The same number of synovial MSCs was also suspended in 400 μL α-MEM medium containing 10% fetal bovine serum (FBS) (5% DMSO, and 1% antibiotic), 95% FBS (and 5% DMSO), or 100% FBS (no DMSO) and cryopreserved at - 80 °C for 7 days. After thawing, the cell suspensions (1.5 μL; 3 × 103 cells) were cultured in 60 cm2 dishes for 14 days for colony formation assays. Additional 62.5 μL samples of cell suspensions (1.25 × 105 cells) were added to tubes and cultured for 21 days for chondrogenesis assays.
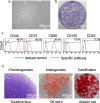
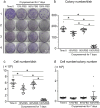
Results: Colony numbers were significantly higher in the Time 0 and 95% FBS groups than in the 10% FBS group (n = 24). Colony numbers were much lower in the 100% FBS group than in the other three groups. The cell numbers per dish reflected the colony numbers. Cartilage pellet weights were significantly heavier in the 95% FBS group than in the 10% FBS group, whereas no difference was observed between the Time 0 and the 95% FBS groups (n = 24). No cartilage pellets formed at all in the 100% FBS group.
Conclusion: Synovial MSCs cryopreserved in 95% FBS with 5% DMSO maintained their colony formation and chondrogenic abilities to the same levels as observed in the cells before cryopreservation. Synovial MSCs cryopreserved in 100% FBS lost their colony formation and chondrogenic abilities.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Complete human serum maintains viability and chondrogenic potential of human synovial stem cells: suitable conditions for transplantation.Stem Cell Res Ther. 2017 Jun 13;8(1):144. doi: 10.1186/s13287-017-0596-0. Stem Cell Res Ther. 2017. PMID: 28610596 Free PMC article.
-
Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum.Arthritis Rheum. 2008 Feb;58(2):501-10. doi: 10.1002/art.23219. Arthritis Rheum. 2008. PMID: 18240254
-
Neuropeptides to replace serum in cryopreservation of mesenchymal stromal cells?Cytotherapy. 2013 Nov;15(11):1385-94. doi: 10.1016/j.jcyt.2013.06.012. Cytotherapy. 2013. PMID: 24094490
-
[Homeostasis and Disorder of Musculoskeletal System.Transplantation of synovial mesenchymal stem cells for cartilage and meniscus regeneration.].Clin Calcium. 2018;28(3):319-327. Clin Calcium. 2018. PMID: 29512522 Review. Japanese.
-
Cryopreservation of Hair-Follicle Associated Pluripotent (HAP) Stem Cells Maintains Differentiation and Hair-Growth Potential.Adv Exp Med Biol. 2016;951:191-198. doi: 10.1007/978-3-319-45457-3_16. Adv Exp Med Biol. 2016. PMID: 27837565 Review.
Cited by
-
Dental-Derived Mesenchymal Stem Cells: State of the Art.Front Cell Dev Biol. 2021 Jun 22;9:654559. doi: 10.3389/fcell.2021.654559. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34239870 Free PMC article. Review.
-
Thawed cryopreserved synovial mesenchymal stem cells show comparable effects to cultured cells in the inhibition of osteoarthritis progression in rats.Sci Rep. 2021 May 6;11(1):9683. doi: 10.1038/s41598-021-89239-8. Sci Rep. 2021. PMID: 33958682 Free PMC article.
-
Comparison of adhesion of thawed and cultured synovial mesenchymal stem cells to the porcine meniscus and the relevance of cell surface microspikes.BMC Mol Cell Biol. 2022 Dec 12;23(1):53. doi: 10.1186/s12860-022-00456-z. BMC Mol Cell Biol. 2022. PMID: 36503422 Free PMC article.
-
Transplantation of human autologous synovial mesenchymal stem cells with trisomy 7 into the knee joint and 5 years of follow-up.Stem Cells Transl Med. 2021 Nov;10(11):1530-1543. doi: 10.1002/sctm.20-0491. Epub 2021 Aug 3. Stem Cells Transl Med. 2021. PMID: 34342383 Free PMC article.
-
Optimisation of cryopreservation conditions, including storage duration and revival methods, for the viability of human primary cells.BMC Mol Cell Biol. 2024 Sep 30;25(1):20. doi: 10.1186/s12860-024-00516-6. BMC Mol Cell Biol. 2024. PMID: 39350017 Free PMC article.
References
-
- Mizuno M, Katano H, Otabe K, Komori K, Matsumoto Y, Fujii S, et al. Platelet-derived growth factor (PDGF)-AA/AB in human serum are potential indicators of the proliferative capacity of human synovial mesenchymal stem cells. Stem Cell Res Ther. 2015;6(1):243. doi: 10.1186/s13287-015-0239-2.. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

