Integrating nanomedicine into clinical radiotherapy regimens
- PMID: 31279729
- PMCID: PMC6745263
- DOI: 10.1016/j.addr.2019.07.002
Integrating nanomedicine into clinical radiotherapy regimens
Abstract
While the advancement of clinical radiotherapy was driven by technological innovations throughout the 20th century, continued improvement relies on rational combination therapies derived from biological insights. In this review, we highlight the importance of combination radiotherapy in the era of precision medicine. Specifically, we survey and summarize the areas of research where improved understanding in cancer biology will propel the field of radiotherapy forward by allowing integration of novel nanotechnology-based treatments.
Keywords: Cancer; Chemoradiotherapy; Dosing; Drug delivery; Multimodal therapy; Nanoparticles; Radiation therapy.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures





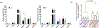

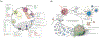

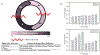

References
-
- Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA. Cancer J. Clin 69 (2019) 7–34. - PubMed
-
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A, Cancer treatment and survivorship statistics, 2016, CA. Cancer J. Clin 66 (2016) 271–289. - PubMed
-
- Palacios Eito A, Cabezas SG, Ugalde PF, del Campo ER, Romero AO, del M Martín MP, Arjona JMR, Paredes MM, Characterization and adequacy of the use of radiotherapy and its trend in time, Radiother. Oncol 106 (2013) 260–265. - PubMed
-
- Delaney G, Jacob S, Featherstone C, Barton M, The role of radiotherapy in cancer treatment, Cancer. 104 (2005) 1129–1137. - PubMed
-
- Barcellos-Hoff MH, Park C, Wright EG, Radiation and the microenvironment – tumorigenesis and therapy, Nat. Rev. Cancer 5 (2005) 867–875. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

