The neurobiological basis for novel experimental therapeutics in dystonia
- PMID: 31279827
- PMCID: PMC6885011
- DOI: 10.1016/j.nbd.2019.104526
The neurobiological basis for novel experimental therapeutics in dystonia
Abstract
Dystonia is a movement disorder characterized by involuntary muscle contractions, twisting movements, and abnormal postures that may affect one or multiple body regions. Dystonia is the third most common movement disorder after Parkinson's disease and essential tremor. Despite its relative frequency, small molecule therapeutics for dystonia are limited. Development of new therapeutics is further hampered by the heterogeneity of both clinical symptoms and etiologies in dystonia. Recent advances in both animal and cell-based models have helped clarify divergent etiologies in dystonia and have facilitated the identification of new therapeutic targets. Advances in medicinal chemistry have also made available novel compounds for testing in biochemical, physiological, and behavioral models of dystonia. Here, we briefly review motor circuit anatomy and the anatomical and functional abnormalities in dystonia. We then discuss recently identified therapeutic targets in dystonia based on recent preclinical animal studies and clinical trials investigating novel therapeutics.
Keywords: Anatomy; Animal models; Basal ganglia; Cerebellum; Drug discovery; Therapy.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of interest: none
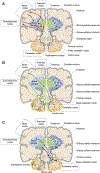
Figures
References
-
- Albin RL, et al., 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–75. - PubMed
-
- Alcantara AA, et al., 2001. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 434, 445–60. - PubMed
-
- Alexander SP, Reddington M, 1989. The cellular localization of adenosine receptors in rat neostriatum. Neuroscience. 28, 645–51. - PubMed
-
- Ambrosio AF, et al., 1996. Modulation of Ca2+ channels by activation of adenosine A1 receptors in rat striatal glutamatergic nerve terminals. Neurosci Lett. 220, 163–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

