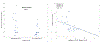Randomized trial of oral cyclophosphamide versus oral cyclophosphamide with celecoxib for recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer
- PMID: 31279962
- PMCID: PMC9018111
- DOI: 10.1016/j.ctarc.2019.100155
Randomized trial of oral cyclophosphamide versus oral cyclophosphamide with celecoxib for recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer
Abstract
Background: Oral metronomic chemotherapy, which has low toxicity, has demonstrated promising anti-tumor and anti-angiogenic properties that may lead to prolonged progression-free survival and improved response rates in patients with recurrent epithelial ovarian cancer (EOC). These effects may be enhanced by the co-administration of anti-angiogenic agents.
Methods: We conducted a randomized phase II clinical trial to evaluate the therapeutic benefit of oral metronomic cyclophosphamide (CTX) alone and with the anti-angiogenic drug celecoxib in patients with gynecological malignancies. 52 patients were randomly assigned to two treatments arms: 50 mg oral CTX daily alone (Arm A) or with 400 mg celecoxib twice daily (Arm B). The primary endpoint was response rate. Secondary endpoints included toxicity, time to treatment failure, and overall survival.
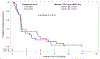
Results: In Arm A (n = 26), 3 patients (12%) had stable disease >6 months and 1 (4%) had a partial response. In Arm B, 5 (19%) had stable disease >6 months and 1 patient (4%) had a partial response. There were no significant between-group differences in overall survival (9.69 months [95% CI 3.84-13.18] vs. 12.55 months [6.67-17.61]) or in median time to treatment failure (1.84 months [1.68-2.76] vs. 1.92 months [1.64-5.22]). The most common adverse events were nausea, vomiting, and abdominal pain.
Conclusions: Oral metronomic CTX has activity with no major toxicities in heavily pretreated recurrent gynecological cancers and may be considered in patients with indolent disease. We did not observe any additional benefit of celecoxib treatment, though this may be due to small sample sizes.
Keywords: Celecoxib; Oral cyclophosphamide; Ovarian cancer.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
References
-
- Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2018. CA: a cancer journal for clinicians, 2018. 68(1): p. 7–30. - PubMed
-
- Folkman J, Tumor angiogenesis: therapeutic implications. New england journal of medicine, 1971. 285(21): p. 1182–1186. - PubMed
-
- Du Bois A, et al., Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials. Cancer, 2009. 115(6): p. 1234–1244. - PubMed
-
- O’Shaughnessy JA, Oral alkylating agents for breast cancer therapy. Drugs, 1999. 58(3): p. 1–9. - PubMed
-
- McGuire WP, et al., Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New England Journal of Medicine, 1996. 334(1): p. 1–6. - PubMed