Efficacy of Neoadjuvant Versus Adjuvant Chemotherapy in Hispanic/Latino (H/L) Women With Local or Locally Advanced Triple-negative Breast Cancer (TNBC)
- PMID: 31280213
- PMCID: PMC6689343
- DOI: 10.21873/invivo.11594
Efficacy of Neoadjuvant Versus Adjuvant Chemotherapy in Hispanic/Latino (H/L) Women With Local or Locally Advanced Triple-negative Breast Cancer (TNBC)
Abstract
Background/aim: The aim of the study was to investigate the efficacy of neoadjuvant and chemotherapy (NACT) and adjuvant chemotherapy (ACT) in Hispanic/Latino (H/L) women with TNBC.
Patients and methods: We reviewed the charts of patients with TNBC, stages I-III, treated at TTUHSC from 2006 to 2016. Overall survival (OS) and recurrence-free survival (RFS) were estimated and compared between the treatment groups. Kaplan-Meier curve and Cox proportional hazards regression analyses were conducted to estimate unadjusted and adjusted effects of NACT compared to ACT.
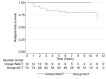
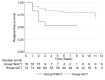
Results: A total of 104 patients with TNBC, 30 (29%) received NACT and 74 (71%) ACT. Women undergoing NACT were younger, with a mean age of 50.8 years. Of the 30 patients who received NACT, 12 (40%) had pathologically complete response (pCR). Women who achieved pCR had an excellent RFS (HR=0.5, p=0.001). Women with residual cancer after NACT had worse outcome compared to patients who received ACT (HR=1.7, p=0.005).
Conclusion: pCR to NACT is a powerful surrogate for OS in H/L women with TNBC.
Keywords: Hispanic/Latino women; Triple-negative breast cancer; adjuvant chemotherapy; locally advancer cancer; neoadjuvant chemotherapy; racial disparity.
Copyright© 2019, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
Philipovskiy Alexander declares that he has no conflict of interest, Corral Javier declares that he has no conflict of interest, Dwivedi Kumar Alok declares that he has no conflict of interest, Heydarian Rosalinda, declares that she has no conflict of interest, Sumit Guar declares that he has no conflict of interest.
Figures
Similar articles
-
Survival outcomes of neoadjuvant versus adjuvant chemotherapy in triple-negative breast cancer: a meta-analysis of 36,480 cases.World J Surg Oncol. 2020 Jun 15;18(1):129. doi: 10.1186/s12957-020-01907-7. World J Surg Oncol. 2020. PMID: 32539858 Free PMC article.
-
Pathological Response and Survival in Triple-Negative Breast Cancer Following Neoadjuvant Carboplatin plus Docetaxel.Clin Cancer Res. 2018 Dec 1;24(23):5820-5829. doi: 10.1158/1078-0432.CCR-18-0585. Epub 2018 Jul 30. Clin Cancer Res. 2018. PMID: 30061361 Free PMC article.
-
Effect of chemotherapy timing in triple-negative breast cancer: a real-world evidence study.Breast Cancer Res Treat. 2025 Jul;212(2):225-236. doi: 10.1007/s10549-025-07716-4. Epub 2025 May 21. Breast Cancer Res Treat. 2025. PMID: 40399665 Free PMC article.
-
The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: a systematic review and meta-analysis.BMC Cancer. 2021 Jan 19;21(1):78. doi: 10.1186/s12885-021-07791-y. BMC Cancer. 2021. PMID: 33468087 Free PMC article.
-
Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis.Ann Oncol. 2018 Jul 1;29(7):1497-1508. doi: 10.1093/annonc/mdy127. Ann Oncol. 2018. PMID: 29873695
Cited by
-
Survival outcomes of neoadjuvant versus adjuvant chemotherapy in triple-negative breast cancer: a meta-analysis of 36,480 cases.World J Surg Oncol. 2020 Jun 15;18(1):129. doi: 10.1186/s12957-020-01907-7. World J Surg Oncol. 2020. PMID: 32539858 Free PMC article.
-
Tumor Lysis Syndrome After a Single Dose of Atezolizumab with Nab-Paclitaxel: A Case Report and Review of Literature.Am J Case Rep. 2020 Sep 16;21:e925248. doi: 10.12659/AJCR.925248. Am J Case Rep. 2020. PMID: 32934194 Free PMC article. Review.
-
Association between tumor mutation profile and clinical outcomes among Hispanic Latina women with triple-negative breast cancer.PLoS One. 2020 Sep 4;15(9):e0238262. doi: 10.1371/journal.pone.0238262. eCollection 2020. PLoS One. 2020. PMID: 32886682 Free PMC article.
-
Examination of Tumor Regression Grading Systems in Breast Cancer Patients Who Received Neoadjuvant Therapy.Pathol Oncol Res. 2020 Oct;26(4):2747-2754. doi: 10.1007/s12253-020-00867-3. Epub 2020 Jul 20. Pathol Oncol Res. 2020. PMID: 32691390 Free PMC article.
-
Comparison of neoadjuvant and adjuvant chemotherapy for operable triple-negative breast cancer before the era of immune checkpoint inhibitors: A retrospective study from the Japanese National Clinical Database-Breast Cancer Registry.Breast. 2025 Jun;81:104460. doi: 10.1016/j.breast.2025.104460. Epub 2025 Mar 25. Breast. 2025. PMID: 40158494 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. PMID: 29313949. DOI: 10.3322/ caac.21442. - PubMed
-
- Miller KD, Goding Sauer A, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero Luna G, Martinez Tyson D, Jemal A, Siegel RL. Cancer statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68:425–445. PMID: 30285281. DOI: 10.3322/caac.21494. - PubMed
-
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. PMID: 17671126. DOI: 10.1158/1078-0432.CCR-06-3045. - PubMed
-
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. PMID: 11773300. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources