A systematic review of the genetic mechanisms of dolutegravir resistance
- PMID: 31280314
- PMCID: PMC6798839
- DOI: 10.1093/jac/dkz256
A systematic review of the genetic mechanisms of dolutegravir resistance
Abstract
Background: Characterizing the mutations selected by the integrase strand transfer inhibitor (INSTI) dolutegravir and their effects on susceptibility is essential for identifying viruses less likely to respond to dolutegravir therapy and for monitoring persons with virological failure (VF) on dolutegravir therapy.
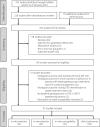
Methods: We systematically reviewed dolutegravir resistance studies to identify mutations emerging under dolutegravir selection pressure, the effect of INSTI resistance mutations on in vitro dolutegravir susceptibility, and the virological efficacy of dolutegravir in antiretroviral-experienced persons.
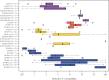
Results and conclusions: We analysed 14 studies describing 84 in vitro passage experiments, 26 studies describing 63 persons developing VF plus INSTI resistance mutations on a dolutegravir-containing regimen, 41 studies describing dolutegravir susceptibility results, and 22 clinical trials and 16 cohort studies of dolutegravir-containing regimens. The most common INSTI resistance mutations in persons with VF on a dolutegravir-containing regimen were R263K, G118R, N155H and Q148H/R, with R263K and G118R predominating in previously INSTI-naive persons. R263K reduced dolutegravir susceptibility ∼2-fold. G118R generally reduced dolutegravir susceptibility >5-fold. The highest levels of reduced susceptibility occurred in viruses containing Q148 mutations in combination with G140 and/or E138 mutations. Dolutegravir two-drug regimens were highly effective for first-line therapy and for virologically suppressed persons provided dolutegravir's companion drug was fully active. Dolutegravir three-drug regimens were highly effective for salvage therapy in INSTI-naive persons provided one or more of dolutegravir's companion drugs was fully active. However, dolutegravir monotherapy in virologically suppressed persons and functional dolutegravir monotherapy in persons with active viral replication were associated with a non-trivial risk of VF plus INSTI resistance mutations.
© The Author(s) 2019. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures
References
-
- Vitoria M, Hill A, Ford N. et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? AIDS 2018; 32: 1551–61. - PubMed
-
- World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. July 2018. HIV Treatment - Interim Guidance https://www.who.int/hiv/pub/guidelines/ARV2018update/en/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

