Deletion of Arginase 2 Ameliorates Retinal Neurodegeneration in a Mouse Model of Multiple Sclerosis
- PMID: 31280447
- PMCID: PMC6857799
- DOI: 10.1007/s12035-019-01691-w
Deletion of Arginase 2 Ameliorates Retinal Neurodegeneration in a Mouse Model of Multiple Sclerosis
Abstract
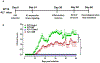
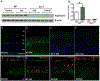
Optic neuritis is a major clinical feature of multiple sclerosis (MS) and can lead to temporary or permanent vision loss. Previous studies from our laboratory have demonstrated the critical involvement of arginase 2 (A2) in retinal neurodegeneration in models of ischemic retinopathy. The current study was undertaken to investigate the role of A2 in MS-mediated retinal neuronal damage and degeneration. Experimental autoimmune encephalomyelitis (EAE) was induced in wild-type (WT) and A2 knockout (A2-/-) mice. EAE-induced motor deficits, loss of retinal ganglion cells, retinal thinning, inflammatory signaling, and glial activation were studied in EAE-treated WT and A2-/- mice and their respective controls. Increased expression of A2 was observed in WT retinas in response to EAE induction. EAE-induced motor deficits were markedly reduced in A2-/- mice compared with WT controls. Retinal flat mount studies demonstrated a significant reduction in the number of RGCs in WT EAE retinas in comparison with normal control mice. A significant improvement in neuronal survival was evident in retinas of EAE-induced A2-/- mice compared with WT. RNA levels of the proinflammatory molecules CCL2, COX2, IL-1α, and IL-12α were significantly reduced in the A2-/- EAE retinas compared with WT EAE. EAE-induced activation of glia (microglia and Müller cells) was markedly reduced in A2-/- retinas compared with WT. Western blot analyses showed increased levels of phospho-ERK1/2 and reduced levels of phospho-BAD in the WT EAE retina, while these changes were prevented in A2-/- mice. In conclusion, our studies establish EAE as an excellent model to study MS-mediated retinal neuronal damage and suggest the potential value of targeting A2 as a therapy to prevent MS-mediated retinal neuronal injury.
Keywords: Arginase 2; EAE; Neurodegeneration; Optic neuritis; Retina; Retinal ganglion cells.
Conflict of interest statement
Conflict of Interest:
The authors declare no conflict of interest.
Figures


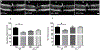




References
-
- Sakai T, Ishihara T, Higaki M, Akiyama G, Tsuneoka H. Therapeutic effect of stealth-type polymeric nanoparticles with encapsulated betamethasone phosphate on experimental autoimmune uveoretinitis. Investigative ophthalmology & visual science. 2011;52:1516–1521 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

