Direct-to-Consumer Hearing Devices: Capabilities, Costs, and Cosmetics
- PMID: 31280709
- PMCID: PMC6614949
- DOI: 10.1177/2331216519858301
Direct-to-Consumer Hearing Devices: Capabilities, Costs, and Cosmetics
Abstract
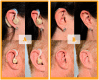
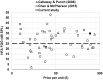
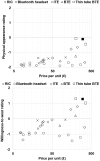
Direct-to-consumer (DTC) hearing devices can be purchased without consulting a hearing health professional. This project aims to compare 28 DTC devices with the most popular hearing aid supplied by the U.K. National Health Service (NHS). The comparison was based on technical performance, cosmetic acceptability, and the ability to match commonly used gain and slope targets. Electroacoustic performance was evaluated in a 2-cc coupler. Match to prescription target for both gain and slope was measured on a Knowles Electronic Manikin for Acoustic Research using a mild and also a moderate sloping hearing loss. Using an online blinded paired comparison of each DTC and the NHS reference device, 126 participants (50 were hearing aid users and 76 were nonhearing aid users) assessed the cosmetic appearance and rated their willingness-to-wear the DTC devices. The results revealed that higher purchase prices were generally associated with a better match to prescribed gain-frequency response shapes, lower distortion, wider bandwidth, better cosmetic acceptability, and higher willingness-to-wear. On every parameter measured, there were devices that performed worse than the NHS device. Most of the devices were rated lower in terms of aesthetic design than the NHS device and provided gain-frequency responses and maximum output levels that were markedly different from those prescribed for commonly encountered audiograms. Because of the absence or inflexibility of most of the devices, they have the potential to deliver poor sound quality and uncomfortably loud sounds. The challenge for manufacturers is to develop low-cost products with cosmetic appeal and appropriate electroacoustic characteristics.
Keywords: direct-to-consumer; gain; hearing aids; personal sound amplification products; slope.
Figures





References
-
- Abrams H., Chisolm T., McManus M., McArdle R. (2012) Initial-fit approach versus verified prescription: Comparing self-perceived hearing aid benefit. Journal of the American Academy of Audiology 23(10): 768–778. doi:10.3766/jaaa.23.10.3. - PubMed
-
- American Hearing Care Associations. (2018). Regulatory recommendation For OTC hearing aids: Safety & effectivness (pp. 1–35). Retrieved from https://www.asha.org/uploadedFiles/Consensus-Paper-From-Hearing-Care-Ass....
-
- ASHA Ad Hoc Committee on Hearing Aid Selection and Fitting (1998) Guidelines for hearing aid fitting for adults. American Journal of Audiology 7(1): 5–13. doi:10.1044/1059-0889.0701.05. - PubMed
-
- Baumfield, A., & Dillon, H. (2001). Factors affecting the use and perceived benefit of ITE and BTE hearing aids. British Journal of Audiology, 35(4), 247–258. doi:10.1080/00305364.2001.11745243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

