IL-10 Dampens the Th1 and Tc Activation through Modulating DC Functions in BCG Vaccination
- PMID: 31281230
- PMCID: PMC6594250
- DOI: 10.1155/2019/8616154
IL-10 Dampens the Th1 and Tc Activation through Modulating DC Functions in BCG Vaccination
Abstract
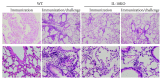
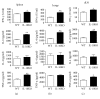
BCG, the only registered vaccine against Mycobacterial Tuberculosis (TB) infection, has been questioned for its protective efficacy for decades. Although lots of efforts were made to improve the BCG antigenicity, few studies were devoted to understand the role of host factors in the variability of the BCG protection. Using the IL-10KO mice and pulmonary tuberculosis infection model, we have addressed the role of IL-10 in the BCG vaccination efficacy. The data showed that IL-10-deficient dendritic cells (DCs) could promote the immune responses through upregulation of the surface costimulatory molecule expression and play an orchestra role through activating CD4+T cell. IL-10-deficient mice had higher IFN γ, TNF α, and IL-6 production after BCG vaccination, which was consistent with the higher proportion of IFN γ +CD3+, IFN γ +CD4+, and IFN γ +CD8+ T cells in the spleen. Particularly, the BCG-vaccinated IL-10KO mice showed less inflammation after TB challenge compared to WT mice, which was supported by the promoted Th1 and Tc, as well as the downregulated Treg responses in IL-10 deficiency. In a conclusion, we demonstrated the negative relationship between Th1/Tc responses with IL-10 production. IL-10 deficiency restored the type 1 immune response through DC activation, which provided better protection against TB infection. Hence, our study offers the first experimental evidence that, contrary to the modulation of BCG, host immunity plays a critical role in the BCG protective efficacy against TB.
Figures






Similar articles
-
Complement C5a anaphylatoxin is an innate determinant of dendritic cell-induced Th1 immunity to Mycobacterium bovis BCG infection in mice.J Leukoc Biol. 2007 Oct;82(4):956-67. doi: 10.1189/jlb.0206119. Epub 2007 Aug 3. J Leukoc Biol. 2007. PMID: 17675563
-
The influence of interferon-β supplemented human dendritic cells on BCG immunogenicity.J Immunol Methods. 2018 Jun;457:15-21. doi: 10.1016/j.jim.2018.03.003. Epub 2018 Mar 6. J Immunol Methods. 2018. PMID: 29522775
-
BCG priming of dendritic cells enhances T regulatory and Th1 function and suppresses allergen-induced Th2 function in vitro and in vivo.Int Arch Allergy Immunol. 2009;150(3):210-20. doi: 10.1159/000222673. Epub 2009 Jun 3. Int Arch Allergy Immunol. 2009. PMID: 19494518
-
Protection against Mycobacterium tuberculosis infection offered by a new multistage subunit vaccine correlates with increased number of IFN-γ+ IL-2+ CD4+ and IFN-γ+ CD8+ T cells.PLoS One. 2015 Mar 30;10(3):e0122560. doi: 10.1371/journal.pone.0122560. eCollection 2015. PLoS One. 2015. PMID: 25822536 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Rethinking Antigen Source: Cancer Vaccines Based on Whole Tumor Cell/tissue Lysate or Whole Tumor Cell.Adv Sci (Weinh). 2023 Aug;10(22):e2300121. doi: 10.1002/advs.202300121. Epub 2023 May 31. Adv Sci (Weinh). 2023. PMID: 37254712 Free PMC article. Review.
-
Protective role of Bacillus Calmette-Guérin vaccine in Alzheimer's disease progression: A systematic review and meta-analysis.Heliyon. 2024 Mar 6;10(5):e27425. doi: 10.1016/j.heliyon.2024.e27425. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38495158 Free PMC article.
-
Expression and purification of Rv2654 recombinant protein from Mycobacterium tuberculosis and its characteristics of immune response.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021 Sept 28;46(9):925-931. doi: 10.11817/j.issn.1672-7347.2021.210318. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34707001 Free PMC article. Chinese, English.
-
Gain‑of‑function of IDO in DCs inhibits T cell immunity by metabolically regulating surface molecules and cytokines.Exp Ther Med. 2023 Apr 3;25(5):234. doi: 10.3892/etm.2023.11933. eCollection 2023 May. Exp Ther Med. 2023. PMID: 37114180 Free PMC article.
-
Acellular Pertussis Vaccines Induce CD8+ and CD4+ Regulatory T Cells That Suppress Protective Tissue-Resident Memory CD4+ T Cells, in Part via IL-10.Eur J Immunol. 2025 Jul;55(7):e51630. doi: 10.1002/eji.202451630. Eur J Immunol. 2025. PMID: 40629997 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

