RUNX2 overexpression and PTEN haploinsufficiency cooperate to promote CXCR7 expression and cellular trafficking, AKT hyperactivation and prostate tumorigenesis
- PMID: 31281490
- PMCID: PMC6587168
- DOI: 10.7150/thno.33292
RUNX2 overexpression and PTEN haploinsufficiency cooperate to promote CXCR7 expression and cellular trafficking, AKT hyperactivation and prostate tumorigenesis
Abstract
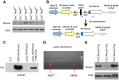
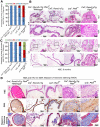
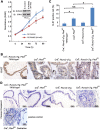
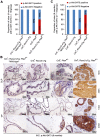
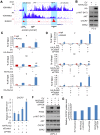
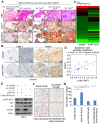
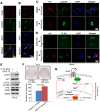
Rationale: The overall success rate of prostate cancer (PCa) diagnosis and therapy has been improved over the years. However, genomic and phenotypic heterogeneity remains a major challenge for effective detection and treatment of PCa. Efforts to better classify PCa into functional subtypes and elucidate the molecular mechanisms underlying prostate tumorigenesis and therapy resistance are warranted for further improvement of PCa outcomes. Methods: We generated Cre+;Runx2-cTg;Ptenp/+ (Runx2-Pten double mutant) mice by crossbreeding Cre+;Runx2-cTg males with Pten conditional (Ptenp/p) females. By using Hematoxylin and Eosin (H&E) staining, SMA and Masson's Trichrome staining, we investigated the effect of PTEN haploinsufficiency in combination with Runx2 overexpression on prostate tumorigenesis. Moreover, we employed immunohistochemistry (IHC) to stain Ki67 for cell proliferation, cleaved caspase 3 for apoptosis and AKT phosphorylation for signaling pathway in prostate tissues. Chromatin immunoprecipitation coupled quantitative PCR (ChIP-qPCR), reverse transcription coupled quantitative PCR (RT-qPCR), western blot (WB) analyses and immunofluorescence (IF) were conducted to determine the underlying mechanism by which RUNX2 regulates CXCR7 and AKT phosphorylation in PCa cells. Results: We demonstrated that mice with prostate-specific Pten heterozygous deletion and Runx2 overexpression developed high-grade prostatic intraepithelial neoplasia (HGPIN) and cancerous lesions at age younger than one year, with concomitant high level expression of Akt phosphorylation and the chemokine receptor Cxcr7 in malignant glands. RUNX2 overexpression induced CXCR7 transcription and membrane location and AKT phosphorylation in PTEN-deficient human PCa cell lines. Increased expression of RUNX2 also promoted growth of PCa cells and this effect was largely mediated by CXCR7. CXCR7 expression also positively correlated with AKT phosphorylation in PCa patient specimens. Conclusions: Our results reveal a previously unidentified cooperative role of RUNX2 overexpression and PTEN haploinsufficiency in prostate tumorigenesis, suggesting that the defined RUNX2-CXCR7-AKT axis can be a viable target for effective treatment of PCa.
Keywords: CXCR7; PTEN; RUNX2; prostate cancer; tumorigenesis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer.J Clin Invest. 2012 May;122(5):1920-32. doi: 10.1172/JCI57477. Epub 2012 Apr 16. J Clin Invest. 2012. PMID: 22505453 Free PMC article.
-
Depletion of SAG/RBX2 E3 ubiquitin ligase suppresses prostate tumorigenesis via inactivation of the PI3K/AKT/mTOR axis.Mol Cancer. 2016 Dec 12;15(1):81. doi: 10.1186/s12943-016-0567-6. Mol Cancer. 2016. PMID: 27955654 Free PMC article.
-
IMP3 accelerates the progression of prostate cancer through inhibiting PTEN expression in a SMURF1-dependent way.J Exp Clin Cancer Res. 2020 Sep 16;39(1):190. doi: 10.1186/s13046-020-01657-0. J Exp Clin Cancer Res. 2020. Retraction in: J Exp Clin Cancer Res. 2023 Jan 17;42(1):24. doi: 10.1186/s13046-023-02599-z. PMID: 32938489 Free PMC article. Retracted.
-
Interplay Among PI3K/AKT, PTEN/FOXO and AR Signaling in Prostate Cancer.Adv Exp Med Biol. 2019;1210:319-331. doi: 10.1007/978-3-030-32656-2_14. Adv Exp Med Biol. 2019. PMID: 31900915 Review.
-
Role of lncRNAs in prostate cancer development and progression.Biol Chem. 2014 Nov 1;395(11):1275-90. doi: 10.1515/hsz-2014-0201. Biol Chem. 2014. PMID: 25153594 Review.
Cited by
-
CXCR7 as a novel therapeutic target for advanced prostate cancer.Oncogene. 2023 Mar;42(11):785-792. doi: 10.1038/s41388-023-02597-7. Epub 2023 Feb 9. Oncogene. 2023. PMID: 36755058 Free PMC article. Review.
-
ZNF521 which is downregulated by miR-802 suppresses malignant progression of Hepatocellular Carcinoma through regulating Runx2 expression.J Cancer. 2020 Aug 1;11(19):5831-5839. doi: 10.7150/jca.45190. eCollection 2020. J Cancer. 2020. PMID: 32913476 Free PMC article.
-
PI3K/AKT inhibition reverses R-CHOP resistance by destabilizing SOX2 in diffuse large B cell lymphoma.Theranostics. 2020 Feb 10;10(7):3151-3163. doi: 10.7150/thno.41362. eCollection 2020. Theranostics. 2020. PMID: 32194860 Free PMC article.
-
Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis.Theranostics. 2019 Oct 18;9(26):8206-8220. doi: 10.7150/thno.37455. eCollection 2019. Theranostics. 2019. PMID: 31754391 Free PMC article.
-
Chemokines and cytokines: Axis and allies in prostate cancer pathogenesis.Semin Cancer Biol. 2022 Nov;86(Pt 3):497-512. doi: 10.1016/j.semcancer.2022.02.017. Epub 2022 Feb 16. Semin Cancer Biol. 2022. PMID: 35181473 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
-
- Wang SI, Parsons R, Ittmann M. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin Cancer Res. 1998;4:811–5. - PubMed
-
- Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D. et al. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–9. - PubMed
-
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG. et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. - PubMed
-
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

