Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy
- PMID: 31281507
- PMCID: PMC6587356
- DOI: 10.7150/thno.33684
Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy
Abstract
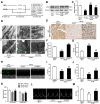
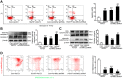
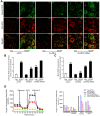
Increasing evidence has implicated the important role of mitochondrial pathology in diabetic cardiomyopathy (DCM), while the underlying mechanism remains largely unclear. The aim of this study was to investigate the role of mitochondrial dynamics in the pathogenesis of DCM and its underlying mechanisms. Methods: Obese diabetic (db/db) and lean control (db/+) mice were used in this study. Mitochondrial dynamics were analyzed by transmission electron microscopy in vivo and by confocal microscopy in vitro. Results: Diabetic hearts from 12-week-old db/db mice showed excessive mitochondrial fission and significant reduced expression of Mfn2, while there was no significant alteration or slight change in the expression of other dynamic-related proteins. Reconstitution of Mfn2 in diabetic hearts inhibited mitochondrial fission and prevented the progression of DCM. In an in-vitro study, cardiomyocytes cultured in high-glucose and high-fat (HG/HF) medium showed excessive mitochondrial fission and decreased Mfn2 expression. Reconstitution of Mfn2 restored mitochondrial membrane potential, suppressed mitochondrial oxidative stress and improved mitochondrial function in HG/HF-treated cardiomyocytes through promoting mitochondrial fusion. In addition, the down-regulation of Mfn2 expression in HG/HF-treated cardiomyocytes was induced by reduced expression of PPARα, which positively regulated the expression of Mfn2 by directly binding to its promoter. Conclusion: Our study provides the first evidence that imbalanced mitochondrial dynamics induced by down-regulated Mfn2 contributes to the development of DCM. Targeting mitochondrial dynamics by regulating Mfn2 might be a potential therapeutic strategy for DCM.
Keywords: Diabetic cardiomyopathy; Mfn2; Mitochondrial dynamics; Mitochondrial dysfunction; PPARα.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures








Similar articles
-
Mitochondrial fusion promoter restores mitochondrial dynamics balance and ameliorates diabetic cardiomyopathy in an optic atrophy 1-dependent way.Acta Physiol (Oxf). 2020 May;229(1):e13428. doi: 10.1111/apha.13428. Epub 2020 Jan 22. Acta Physiol (Oxf). 2020. PMID: 31840416
-
Nicotinamide riboside promotes Mfn2-mediated mitochondrial fusion in diabetic hearts through the SIRT1-PGC1α-PPARα pathway.Free Radic Biol Med. 2022 Apr;183:75-88. doi: 10.1016/j.freeradbiomed.2022.03.012. Epub 2022 Mar 19. Free Radic Biol Med. 2022. PMID: 35318101
-
Ginsenoside Rb1 ameliorates lipotoxicity-induced myocardial injury in diabetes mellitus by regulating Mfn2.Eur J Pharmacol. 2024 Jul 5;974:176609. doi: 10.1016/j.ejphar.2024.176609. Epub 2024 Apr 25. Eur J Pharmacol. 2024. PMID: 38677536
-
[Role of mitochondrial dynamics in diabetic cardiomyopathy and regulatory mechanisms].Sheng Li Xue Bao. 2024 Feb 25;76(1):128-136. Sheng Li Xue Bao. 2024. PMID: 38444138 Review. Chinese.
-
Mitochondrial quality control in diabetic cardiomyopathy: from molecular mechanisms to therapeutic strategies.Int J Biol Sci. 2022 Aug 15;18(14):5276-5290. doi: 10.7150/ijbs.75402. eCollection 2022. Int J Biol Sci. 2022. PMID: 36147470 Free PMC article. Review.
Cited by
-
A comprehensive review of the novel therapeutic targets for the treatment of diabetic cardiomyopathy.Ther Adv Cardiovasc Dis. 2023 Jan-Dec;17:17539447231210170. doi: 10.1177/17539447231210170. Ther Adv Cardiovasc Dis. 2023. PMID: 38069578 Free PMC article. Review.
-
Mitochondria-Endoplasmic Reticulum Contacts: The Promising Regulators in Diabetic Cardiomyopathy.Oxid Med Cell Longev. 2022 Apr 11;2022:2531458. doi: 10.1155/2022/2531458. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35450404 Free PMC article. Review.
-
Withaferin A Enhances Mitochondrial Biogenesis and BNIP3-Mediated Mitophagy to Promote Rapid Adaptation to Extreme Hypoxia.Cells. 2022 Dec 25;12(1):85. doi: 10.3390/cells12010085. Cells. 2022. PMID: 36611879 Free PMC article.
-
Heart failure in diabetes.Metabolism. 2021 Dec;125:154910. doi: 10.1016/j.metabol.2021.154910. Epub 2021 Oct 8. Metabolism. 2021. PMID: 34627874 Free PMC article. Review.
-
Mitophagy in Diabetic Cardiomyopathy: Roles and Mechanisms.Front Cell Dev Biol. 2021 Sep 27;9:750382. doi: 10.3389/fcell.2021.750382. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34646830 Free PMC article. Review.
References
-
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. - PubMed
-
- Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–6. - PubMed
-
- Nathan DM. Diabetes: Advances in Diagnosis and Treatment. Jama. 2015;314:1052–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

