Polydopamine coated multifunctional lanthanide theranostic agent for vascular malformation and tumor vessel imaging beyond 1500 nm and imaging-guided photothermal therapy
- PMID: 31281519
- PMCID: PMC6587345
- DOI: 10.7150/thno.31864
Polydopamine coated multifunctional lanthanide theranostic agent for vascular malformation and tumor vessel imaging beyond 1500 nm and imaging-guided photothermal therapy
Abstract
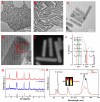
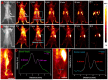
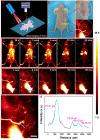
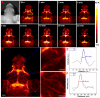
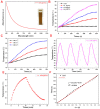
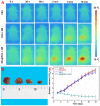
The optical imaging guided tumor vessels and vascular malformation visualization by using the second near infrared emission beyond 1500 nm (NIR-II) is emerged as the next generation fluorescence imaging technique for early tumor diagnosis and identification of tumor-associated vascular features. On the other hand, developing theranostic probes for NIR-II imaging guided photothermal therapy (PTT) is of great significance, which is rarely explored. Herein, a high performance theranostic nanoplatform based on the core-shell structured NaLuF4 nanorods@polydopamine (denoted as NRs@PDA) by integrating the new advanced NIR-II imaging beyond 1500 nm with PTT function was developed for tumor-associated vascular malformation visualization and imaging-guided PTT. Methods: In this work, the hydrophilic NaLuF4 NRs@PDA therapeutic probe was synthesized by using a reverse microemulsion method. The crystal phase, morphology, emission spectra and photothermal performance of the synthesized samples were systematically characterized. The NIR-II optical imaging and photothermal properties were investigated by in vitro and in vivo experiments. Results: The NaLuF4 NRs@PDA therapeutic probe possessed efficient NIR-II emission centered at 1525 nm with high quantum yield (QY), good photo-stability and high biocompatibility. In vivo NIR-IIb imaging based on the designed probe can clearly visualize the whole-body vessel and brain vessel with high spatial resolution, especially tumor-associated vessels. In addition, in vitro and in vivo experiments also demonstrated that the designed NaLuF4 NRs@PDA probe possessed efficient photothermal conversion efficiency (40.18%) for PTT ablation of tumor. Conclusion: With the excellent NIR-II imaging ability and PTT of tumor, the designed theranostic nanoplatform successfully realize the simultaneous tumor vessel diagnosis and tumor therapy, which may provide the opportunity of designing new theranostic bioprobes with combination of the NIR-II optical imaging technique and PTT function for tumor diagnosis and therapy.
Keywords: Lanthanide nanorods; photothermal therapy; theranostic nanoplatform; tumor angiography.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor.Acta Biomater. 2017 Jan 1;47:124-134. doi: 10.1016/j.actbio.2016.10.010. Epub 2016 Oct 6. Acta Biomater. 2017. PMID: 27721008
-
Non-Invasive Optical Guided Tumor Metastasis/Vessel Imaging by Using Lanthanide Nanoprobe with Enhanced Down-Shifting Emission beyond 1500 nm.ACS Nano. 2019 Jan 22;13(1):248-259. doi: 10.1021/acsnano.8b05431. Epub 2019 Jan 7. ACS Nano. 2019. PMID: 30604961
-
Mussel-Inspired Polydopamine-Coated Lanthanide Nanoparticles for NIR-II/CT Dual Imaging and Photothermal Therapy.ACS Appl Mater Interfaces. 2017 Aug 16;9(32):26674-26683. doi: 10.1021/acsami.7b06109. Epub 2017 Aug 2. ACS Appl Mater Interfaces. 2017. PMID: 28726368
-
Functionalized theranostic nanocarriers with bio-inspired polydopamine for tumor imaging and chemo-photothermal therapy.J Control Release. 2019 Sep 10;309:203-219. doi: 10.1016/j.jconrel.2019.07.036. Epub 2019 Jul 27. J Control Release. 2019. PMID: 31362077 Review.
-
Applications of nanotheranostics in the second near-infrared window in bioimaging and cancer treatment.Nanoscale. 2024 Dec 5;16(47):21697-21730. doi: 10.1039/d4nr03058c. Nanoscale. 2024. PMID: 39508492 Review.
Cited by
-
Nanoparticles Design for Theranostic Approach in Cancer Disease.Cancers (Basel). 2022 Sep 24;14(19):4654. doi: 10.3390/cancers14194654. Cancers (Basel). 2022. PMID: 36230578 Free PMC article. Review.
-
Phase-specific and laser-modulated polydopamine-chlorella-curdlan hydrogels: pioneering a melanoma integrative therapy.Theranostics. 2025 Jun 23;15(15):7627-7652. doi: 10.7150/thno.113417. eCollection 2025. Theranostics. 2025. PMID: 40756368 Free PMC article.
-
Excretable IR-820 for in vivo NIR-II fluorescence cerebrovascular imaging and photothermal therapy of subcutaneous tumor.Theranostics. 2019 Aug 9;9(19):5706-5719. doi: 10.7150/thno.31332. eCollection 2019. Theranostics. 2019. PMID: 31534513 Free PMC article.
-
808 nm light triggered lanthanide nanoprobes with enhanced down-shifting emission beyond 1500 nm for imaging-guided resection surgery of tumor and vascular visualization.Theranostics. 2020 May 23;10(15):6875-6885. doi: 10.7150/thno.41967. eCollection 2020. Theranostics. 2020. PMID: 32550909 Free PMC article.
-
Recent Progress in NIR-II Contrast Agent for Biological Imaging.Front Bioeng Biotechnol. 2020 Jan 30;7:487. doi: 10.3389/fbioe.2019.00487. eCollection 2019. Front Bioeng Biotechnol. 2020. PMID: 32083067 Free PMC article. Review.
References
-
- He F, Yang GX, Yang PP, Yu YX, Lv RC, Li CX. et al. A new single 808 nm NIR light-induced imaging-guided multifunctional cancer therapy platform. Adv Funct Mater. 2015;25:3966–76.
-
- Wang YH, Wang HG, Liu DP, Song SY, Wang X, Zhang HJ. Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials. 2013;34:7715–24. - PubMed
-
- Cheng L, Yang K, Li YG, Chen JH, Wang C, Shao MW. et al. Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy. Angew Chem Int Ed Engl. 2011;50:7385–90. - PubMed
-
- Xiao QF, Zheng XP, Bu WB, Ge WQ, Zhang SJ, Chen F. et al. A core/satellite multifunctional nanotheranostic for in vivo imaging and tumor eradication by radiation/photothermal synergistic therapy. J Am Chem Soc. 2013;135:13041–8. - PubMed
-
- Dong K, Liu Z, Li ZH, Ren JS, Qu XG. Hydrophobic anticancer drug delivery by a 980 nm laser-driven photothermal vehicle for efficient synergistic therapy of cancer cells in vivo. Adv Mater. 2013;25:4452–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

