Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo
- PMID: 31281533
- PMCID: PMC6592170
- DOI: 10.7150/thno.33638
Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo
Abstract
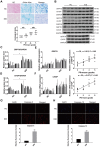
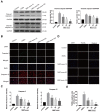
Objectives: Intervertebral disc degeneration (IDD) is widely accepted as a cause of low back pain and related degenerative musculoskeletal disorders. Nucleus pulposus (NP) cell apoptosis which is related to excessive endoplasmic reticulum (ER) stress in the intervertebral disc (IVD) could aggravate IDD progression. Many studies have shown the therapeutic potential of exosomes derived from bone marrow mesenchymal stem cells (MSC-exos) in degenerative diseases. We hypothesized that the delivery of MSC-exos could modulate ER stress and inhibit excessive NP cell apoptosis during IDD. Methods: The ER stress levels were measured in normal or degenerative NP tissues for contrast. The effects of MSC-exos were testified in advanced glycation end products (AGEs) -induced ER stress in human NP cells. The mechanism involving AKT and ERK signaling pathways was investigated using RNA interference or signaling inhibitors. Histological or immunohistochemical analysis and TUNEL staining were used for evaluating MSC-exos therapeutic effects in vivo. Results: The ER stress level and apoptotic rate was elevated in degenerative IVD tissues. MSC-exos could attenuate ER stress-induced apoptosis by activating AKT and ERK signaling. Moreover, delivery of MSC-exos in vivo modulated ER stress-related apoptosis and retarded IDD progression in a rat tail model. Conclusions: These results highlight the therapeutic effects of exosomes in preventing IDD progression. Our work is the first to demonstrate that MSC-exos could modulate ER stress-induced apoptosis during AGEs-associated IVD degeneration.
Keywords: Mesenchymal stem cells; advanced glycation end products; endoplasmic reticulum stress; exosomes; intervertebral disc degeneration.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Priyadarshani P, Li Y, Yao L. Advances in biological therapy for nucleus pulposus regeneration. Osteoarthritis Cartilage. 2016;24:206–12. - PubMed
-
- Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249–64. - PubMed
-
- Vergroesen PP, Kingma I, Emanuel KS, Hoogendoorn RJ, Welting TJ, van Royen BJ. et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23:1057–70. - PubMed
-
- Nowotny K, Castro JP, Hugo M, Braune S, Weber D, Pignitter M. et al. Oxidants produced by methylglyoxal-modified collagen trigger ER stress and apoptosis in skin fibroblasts. Free Radic Biol Med. 2018;120:102–13. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

