Gadolinium-Chelated Conjugated Polymer-Based Nanotheranostics for Photoacoustic/Magnetic Resonance/NIR-II Fluorescence Imaging-Guided Cancer Photothermal Therapy
- PMID: 31281539
- PMCID: PMC6592180
- DOI: 10.7150/thno.34390
Gadolinium-Chelated Conjugated Polymer-Based Nanotheranostics for Photoacoustic/Magnetic Resonance/NIR-II Fluorescence Imaging-Guided Cancer Photothermal Therapy
Abstract
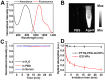
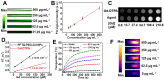
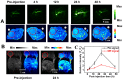
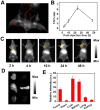
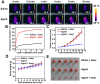
Our exploiting versatile multimodal theranostic agent aims to integrate the complementary superiorities of photoacoustic imaging (PAI), second near-infrared (NIR-II, 1000-1700) fluorescence and T1-weighted magnetic resonance imaging (MRI) with an ultimate objective of perfecting cancer diagnosis, thus improving cancer therapy efficacy. Herein, we engineered and prepared a water-soluble gadolinium-chelated conjugated polymer-based theranostic nanomedicine (PFTQ-PEG-Gd NPs) for in vivo tri-mode PA/MR/NIR-II imaging-guided tumor photothermal therapy (PTT). Methods: We firstly constructed a semiconducting polymer composed of low-bandgap donor-acceptor (D-A) which afforded the strong NIR absorption for PAI/PTT and long fluorescence emission to NIR-II region for in vivo imaging. Then, the remaining carboxyl groups of the polymeric NPs could effectively chelate with Gd3+ ions for MRI. The in vitro characteristics of the PFTQ-PEG-Gd NPs were studied and the in vivo multimode imaging as well as anti-tumor efficacy of the NPs was evaluated using 4T1 tumor-bearing mice. Results: The obtained theranostic agent showed excellent chemical and optical stability as well as low biotoxicity. After 24 h of systemic administration using PQTF-PEG-Gd NPs, the tumor sites of living mice exhibited obvious enhancement in PA, NIR-II fluorescence and positive MR signal intensities. Better still, a conspicuous tumor growth restraint was detected under NIR light irradiation after administration of PQTF-PEG-Gd NPs, indicating the efficient photothermal potency of the nano-agent. Conclusion: we triumphantly designed and synthesized a novel and omnipotent semiconducting polymer nanoparticles-based theranostic platform for PAI, NIR-II fluorescence imaging as well as positive MRI-guided tumor PTT in living mice. We expect that such a novel organic nano-platform manifests a great promise for high spatial resolution and deep penetration cancer theranostics.
Keywords: conjugated polymer; magnetic resonance imaging; photoacoustic imaging; photothermal therapy; second near-infrared fluorescence imaging.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Copper Manganese Sulfide Nanoplates: A New Two-Dimensional Theranostic Nanoplatform for MRI/MSOT Dual-Modal Imaging-Guided Photothermal Therapy in the Second Near-Infrared Window.Theranostics. 2017 Oct 17;7(19):4763-4776. doi: 10.7150/thno.21694. eCollection 2017. Theranostics. 2017. PMID: 29187902 Free PMC article.
-
Semiconducting polymer nanotheranostics for NIR-II/Photoacoustic imaging-guided photothermal initiated nitric oxide/photothermal therapy.Biomaterials. 2019 Oct;217:119304. doi: 10.1016/j.biomaterials.2019.119304. Epub 2019 Jun 25. Biomaterials. 2019. PMID: 31279099
-
Chemotherapeutic drug-photothermal agent co-self-assembling nanoparticles for near-infrared fluorescence and photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy.J Control Release. 2017 Jul 28;258:95-107. doi: 10.1016/j.jconrel.2017.05.011. Epub 2017 May 10. J Control Release. 2017. PMID: 28501673
-
Organic Nanotheranostics for Photoacoustic Imaging-Guided Phototherapy.Curr Med Chem. 2019;26(8):1389-1405. doi: 10.2174/0929867324666170921103152. Curr Med Chem. 2019. PMID: 28933283 Review.
-
Organic Semiconductors for Photothermal Therapy and Photoacoustic Imaging.Chembiochem. 2019 Jul 1;20(13):1628-1636. doi: 10.1002/cbic.201800818. Epub 2019 Apr 18. Chembiochem. 2019. PMID: 30690811 Review.
Cited by
-
Grafted semiconducting polymer amphiphiles for multimodal optical imaging and combination phototherapy.Chem Sci. 2020 Jul 15;11(39):10553-10570. doi: 10.1039/d0sc01721c. Chem Sci. 2020. PMID: 34094312 Free PMC article. Review.
-
Progress of near-infrared-II fluorescence in precision diagnosis and treatment of colorectal cancer.Heliyon. 2023 Dec 3;9(12):e23209. doi: 10.1016/j.heliyon.2023.e23209. eCollection 2023 Dec. Heliyon. 2023. PMID: 38149207 Free PMC article. Review.
-
TADF-based NIR-II semiconducting polymer dots for in vivo 3D bone imaging.Chem Sci. 2022 Aug 18;13(34):10074-10081. doi: 10.1039/d2sc03271f. eCollection 2022 Aug 31. Chem Sci. 2022. PMID: 36128252 Free PMC article.
-
Extrahepatic cholangiography in near-infrared II window with the clinically approved fluorescence agent indocyanine green: a promising imaging technology for intraoperative diagnosis.Theranostics. 2020 Feb 19;10(8):3636-3651. doi: 10.7150/thno.41127. eCollection 2020. Theranostics. 2020. PMID: 32206113 Free PMC article.
-
NIR-II fluorescence microscopic imaging of cortical vasculature in non-human primates.Theranostics. 2020 Mar 4;10(9):4265-4276. doi: 10.7150/thno.43533. eCollection 2020. Theranostics. 2020. PMID: 32226552 Free PMC article.
References
-
- Li J, Pu K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem Soc Rev. 2019;48:38–71. - PubMed
-
- Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional Nanomaterials for Phototherapies of Cancer. Chem Rev. 2014;114:10869–10939. - PubMed
-
- Ma L, Liu Y, Liu L, Jiang A, Mao F, Zhou J. et al. Simultaneous Activation of Short-Wave Infrared (SWIR) Light and Paramagnetism by a Functionalized Shell for High Penetration and Spatial Resolution Theranostics. Adv Funct Mater. 2018;28:1705057.
-
- Ge J, Jia Q, Liu W, Guo L, Liu Q, Lan M. et al. Red-Emissive Carbon Dots for Fluorescent, Photoacoustic, and Thermal Theranostics in Living Mice. Adv Mater. 2015;27:4169–4177. - PubMed
-
- Guo B, Sheng Z, Hu D, Li A, Xu S, Manghnani P. et al. Molecular Engineering of Conjugated Polymers for Biocompatible Organic Nanoparticles with Highly Efficient Photoacoustic and Photothermal Performance in Cancer Theranostics. ACS Nano. 2017;11:10124–10134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

