Premature dissolution of the Agile patency device: implications for capsule endoscopy
- PMID: 31281621
- PMCID: PMC6583767
- DOI: 10.1136/flgastro-2018-101112
Premature dissolution of the Agile patency device: implications for capsule endoscopy
Abstract
Background: The main risk of capsule endoscopy is retention of the capsule behind a stricture. Passage of an intact Agile patency device (Medtronic, Dublin, Ireland) through the small bowel is widely used to ensure luminal patency, although capsule retention has occurred in patients who have had a reassuring patency study. The device is designed to remain intact for at least 30 hours postingestion, such that loss of signal from the radiofrequency identification tag contained within, or absence of the device on radiological imaging, implies unimpeded intestinal transit.
Aim: To identify the rate of premature dissolution (<30 hours postingestion) of the Agile patency device.
Methods: Outcomes of all consecutive patients having an Agile patency device were analysed.
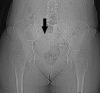
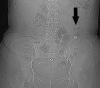
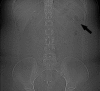
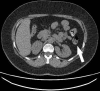
Results: Premature dissolution of the patency device occurred in 5 of 307 patients, an incidence of 1.3%. This was recognised by the detection of a persistent radiofrequency signal after radiological imaging had failed to identify the patency device, prompting a careful search for the radiofrequency tag on the CT scout film. The tag was difficult to detect because of an oblique lie making it appear smaller than its 13×3 mm size and confusion with intra-abdominal or other metallic fragments.
Conclusions: In the absence of radiological evidence of an intact Agile patency device, premature dissolution should be suspected in patients registering a persistent radiofrequency signal and confirmed by identifying the radiofrequency identification tag. Failure to do so might result in false reassurance that capsule endoscopy could be performed without risk of retention.
Keywords: diagnostic and therapeutic endoscopy; endoscopy; small bowel disease; small bowel enteroscopy.
Conflict of interest statement
Competing interests: MMcA has received financial support for research and conference attendance from Given Imaging Ltd, Intromedic Ltd and Ankon Ltd; research support from Jinshan Science and Technology Ltd and has acted as a consultant for Medtronic Ltd. NW, AH, VT, MFH, RS and TB have no conflicts of interests or financial ties to disclose.
Figures


References
-
- Rondonotti E, Soncini M, Girelli CM, et al. . Short article: Negative small-bowel cross-sectional imaging does not exclude capsule retention in high-risk patients. Eur J Gastroenterol Hepatol 2016;28:871–5. - PubMed
LinkOut - more resources
Full Text Sources
