Murine Model Study of a New Receptor-Targeted Tracer for Sentinel Lymph Node in Breast Cancer
- PMID: 31281729
- PMCID: PMC6597406
- DOI: 10.4048/jbc.2019.22.e28
Murine Model Study of a New Receptor-Targeted Tracer for Sentinel Lymph Node in Breast Cancer
Abstract
Purpose: Sentinel lymph node biopsy (SLNB), a critical staging and treatment step, has replaced axillary lymph node (LN) dissection as the standard staging procedure for early stage breast cancer patients with clinically negative axillary LNs. Hence, using a murine sentinel lymph node (SLN) model, we investigated the localization effect of the new receptor-targeted tracer, indocyanine green (ICG)-rituximab, on breast cancer SLNB.
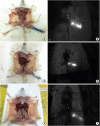
Methods: After establishing the murine SLN model, different doses of ICG-rituximab were subcutaneously injected into the hind insteps of BALB/c mice to determine the optimal dose and imaging time using continuous (> 3 hours) MDM-I fluorescence vasculature imaging. To explore the capacity of ICG-rituximab for sustained SLN localization with the optimal dose, MDM-I imaging was monitored at 6, 12, and 24 hours.
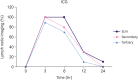
Results: The popliteal LN was defined as the SLN for hindlimb lymphatic drainage, the iliac LN as the secondary, and the para-aortic or renal LN as the tertiary LNs. The SLN initial imaging and optimal imaging times were shortened with increased ICG-rituximab doses, and the imaging rates of the secondary and tertiary LNs increased accordingly. The optimal ICG dose was 0.12 μg, and its optimal imaging time was 34 minutes. After 24 hours, the SLN imaging rate remained 100%, while those of the secondary and the tertiary LNs increased from 0% (6 hours) and 0% (6 hours) to 10% (12 hours) and 10% (12 hours) to 20% (24 hours) and 10% (24 hours), respectively.
Conclusion: ICG-rituximab localized to the SLN without imaging from the secondary or tertiary LNs within 6 hours. The optimal ICG dose was 0.12 μg, and the optimal interval for SLN detection was 34 minutes to 6 hours post-injection. This novel receptor-targeted tracer is of great value to clinical research and application.
Keywords: Animal model; Indocyanine green; Rituximab; Sentinel lymph node; Synthetic imaging agent.
Conflict of interest statement
Conflict of Interest: The authors declare that they have no competing interests.
Figures



References
-
- Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365–1383. - PubMed
-
- Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. - PubMed
-
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. - PubMed
-
- Zavagno G, De Salvo GL, Scalco G, Bozza F, Barutta L, Del Bianco P, et al. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008;247:207–213. - PubMed
-
- Hirche C, Murawa D, Mohr Z, Kneif S, Hünerbein M. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010;121:373–378. - PubMed
LinkOut - more resources
Full Text Sources

