Endocrine and Targeted Therapy for Hormone-Receptor-Positive, HER2-Negative Advanced Breast Cancer: Insights to Sequencing Treatment and Overcoming Resistance Based on Clinical Trials
- PMID: 31281796
- PMCID: PMC6597942
- DOI: 10.3389/fonc.2019.00510
Endocrine and Targeted Therapy for Hormone-Receptor-Positive, HER2-Negative Advanced Breast Cancer: Insights to Sequencing Treatment and Overcoming Resistance Based on Clinical Trials
Abstract
Background: Advanced hormone-receptor positive HER2 negative breast cancer is a common and a very heterogeneous disease. Hormone therapy is the main first line treatment of choice, given alone or in combination with other agents that have shown to improve patient outcomes, Nevertheless, treatment remains generally palliative rather than curative. Sequencing of such treatment remains challenging, especially with resurgence of variable resistance patterns. Multiple attempts have been made to overcome resistance and improve patient survival, yet resistance remains not very well understood and metastatic cancer remains a disease with dismal prognosis. Methods: In this paper, we searched pubmed database as well as local and international meetings for all studies discussing advanced and metastatic hormone-receptor-positive, her2-negative breast cancer, hormonal treatment, resistance to hormonal treatment, mechanism of resistance, and means to overcome such resistance. Conclusion: There does not exist an optimal treatment sequence for hormone-receptor-positive, her2-negative advanced breast cancer. However, after review of literature, a reasonable approach may be starting with tamoxifen, aromatase inhibitors, or fulvestrant in absence of visceral crisis, in addition to ensuring adequate ovarian function suppression in pre/peri-menopausal women. Aromatase inhibitors and fulvestrant seem to be superior. Resistance to such agents is increasing, mostly attributed to genetic and molecular changes. Multiple modalities are addressed to overcome such resistance including use of CKD4/6 inhibitors, mTOR inhibitors and PI3K inhibitors in addition to other agents under study, all with promising results. CDK4/6 inhibitors work best when used in frontline setting. Finally, treatment of breast cancer remains a growing field, and more studies are to be awaited.
Keywords: HER2 negative; advanced breast cancer; endocrine resistance; endocrine therapy; hormone receptor positive; overcoming resistance; sequencing treatment.
Figures

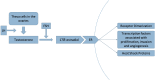
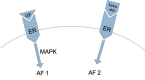
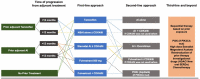
References
-
- Cancer Genome Atlas Network Breast Cancer. Estimated Incidence, Mortality and Prevalence Worldwide in 2012. International Agency for Research on Cancer (2012). Available online at: http://globocan.iarc.fr/Pages/factsheetscancer.aspx
-
- Bethesda M. SEER Cancer Stat Facts: Female Breast Cancer Subtypes. 18 ed. National Cancer Institute (2018).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

